Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses
- PMID: 10510079
- PMCID: PMC2195643
- DOI: 10.1084/jem.190.7.895
Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses
Abstract
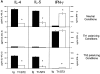
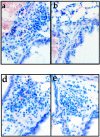
T1/ST2 is an orphan receptor of unknown function that is expressed on the surface of murine T helper cell type 2 (Th2), but not Th1 effector cells. In vitro blockade of T1/ST2 signaling with an immunoglobulin (Ig) fusion protein suppresses both differentiation to and activation of Th2, but not Th1 effector populations. In a nascent Th2-dominated response, anti-T1/ST2 monoclonal antibody (mAb) inhibited eosinophil infiltration, interleukin 5 secretion, and IgE production. To determine if these effects were mediated by a direct effect on Th2 cells, we next used a murine adoptive transfer model of Th1- and Th2-mediated lung mucosal immune responses. Administration of either T1/ST2 mAb or T1/ST2-Ig abrogated Th2 cytokine production in vivo and the induction of an eosinophilic inflammatory response, but failed to modify Th1-mediated inflammation. Taken together, our data demonstrate an important role of T1/ST2 in Th2-mediated inflammatory responses and suggest that T1/ST2 may prove to be a novel target for the selective suppression of Th2 immune responses.
Figures







References
-
- Ho I.C., Hodge M.R., Rooney J.W., Glimcher L.H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. - PubMed
-
- Zheng W., Flavell R. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. - PubMed
-
- Zhang D.-H., Cohn L., Ray P., Bottomly K., Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 1997;272:21597–21603. - PubMed
-
- Ouyang W., Ranganath S.H., Weindel K., Bhattacharya D., Murphy T.L., Sha W.C., Murphy K.M. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. - PubMed
-
- Sallusto F., Mackay C.R., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. - PubMed

