Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice
- PMID: 10510081
- PMCID: PMC2195652
- DOI: 10.1084/jem.190.7.915
Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice
Abstract
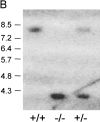
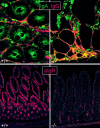
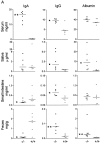
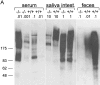
Mucosal surfaces are protected specifically by secretory immunoglobulin A (SIgA) and SIgM generated through external translocation of locally produced dimeric IgA and pentameric IgM. Their active transport is mediated by the epithelial polymeric Ig receptor (pIgR), also called the transmembrane secretory component. Paracellular passive external transfer of systemic and locally produced antibodies also provides mucosal protection, making the biological importance of secretory immunity difficult to assess. Here we report complete lack of active external IgA and IgM translocation in pIgR knockout mice, indicating no redundancy in epithelial transport mechanisms. The knockout mice were of normal size and fertility but had increased serum IgG levels, including antibodies to Escherichia coli, suggesting undue triggering of systemic immunity. Deterioration of their epithelial barrier function in the absence of SIgA (and SIgM) was further attested to by elevated levels of albumin in their saliva and feces, reflecting leakage of serum proteins. Thus, SIgA did not appear to be essential for health under the antigen exposure conditions of these experimental animals. Nevertheless, our results showed that SIgA contributes to maintenance of mucosal homeostasis. Production of SIgA might therefore be a variable in the initiation of human immunopathology such as inflammatory bowel disease or gluten-sensitive enteropathy.
Figures




References
-
- Brandtzaeg P. History of oral tolerance and mucosal immunity. Ann. NY Acad. Sci. 1996;778:1–27. - PubMed
-
- Mestecky J., Moro I., Underdown B. Mucosal immunoglobulins. In: Mestecky J., Bienstock J., McGhee J., Lamm M., Strober W., Ogra P., editors. Mucosal Immunology. Academic Press; San Diego, CA: 1999. p. 133.
-
- Stokes C.R., Soothill J.F., Turner M.W. Immune exclusion is a function of IgA. Nature. 1975;255:745–746. - PubMed
-
- Mazanec M.B., Nedrud J.G., Kaetzel C.S., Lamm M.E. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today. 1993;14:430–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

