Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo. A role for granulocyte/macrophage colony-stimulating factor
- PMID: 10510082
- PMCID: PMC2195653
- DOI: 10.1084/jem.190.7.923
Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo. A role for granulocyte/macrophage colony-stimulating factor
Abstract
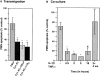
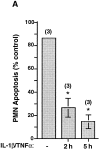
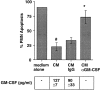
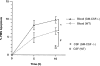
The activation of endothelium is important in recruiting neutrophils to sites of inflammation and in modulating their function. We demonstrate that conditioned medium from cultured, activated endothelial cells acts to significantly delay the constitutive apoptosis of neutrophils, resulting in their enhanced survival and increased phagocytic function. The antiapoptotic activity is, in part, attributable to granulocyte/macrophage colony-stimulating factor (GM-CSF) secreted by activated endothelial cells. The in vivo relevance of these findings was investigated in a cytokine-induced model of acute meningitis in mice. Peripheral blood neutrophils (PBNs) from mice with meningitis exhibited a delay in apoptosis compared with untreated mice. Furthermore, neutrophils recovered from the inflamed cerebrospinal fluid (CSF) exhibited enhanced survival compared with neutrophils isolated from the peripheral blood of the same animals. In unchallenged GM-CSF-deficient mice, the apoptosis of circulating PBNs was similar to wild-type animals; however, after cytokine-induced meningitis, the delay in neutrophil apoptosis typically observed in wild-type mice was attenuated. In contrast, the apoptosis of neutrophils recovered from the CSF of mice of both genotypes was comparable. Taken together, these studies suggest that neutrophil apoptosis is regulated during an inflammatory response, in both intravascular and extravascular compartments. GM-CSF released by activated endothelium can act to increase neutrophil survival and function in the peripheral blood, whereas other factor(s) appear to perform this function in the extravascular space.
Figures




References
-
- Bicknell S., van Eeden S., Hayashi S., Hards J., English D., Hogg J.C. A non-radioisotopic method for tracing neutrophils in vivo using 5′-bromo-2′-deoxyuridine. Am. J. Respir. Cell Mol. Biol. 1994;10:16–23. - PubMed
-
- Savill J., Haslett C. Fate of neutrophils. In: Hellewell P.G., Williams T.J., editors. Immunopharmacology of Neutrophils. Academic Press, Inc; San Diego: 1994. pp. 295–314.
-
- Whyte M.K.B., Meagher L.C., MacDermot J., Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 1993;150:5124–5134. - PubMed
-
- Dransfield I., Stocks S.C., Haslett C. Regulation of cell adhesion expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–3273. - PubMed
-
- Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

