Receptor avidity and costimulation specify the intracellular Ca2+ signaling pattern in CD4(+)CD8(+) thymocytes
- PMID: 10510084
- PMCID: PMC2195644
- DOI: 10.1084/jem.190.7.943
Receptor avidity and costimulation specify the intracellular Ca2+ signaling pattern in CD4(+)CD8(+) thymocytes
Abstract
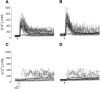
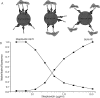
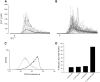
Thymocyte maturation is governed by antigen-T cell receptor (TCR) affinity and the extent of TCR aggregation. Signals provided by coactivating molecules such as CD4 and CD28 also influence the fate of immature thymocytes. The mechanism by which differences in antigen-TCR avidity encode unique maturational responses of lymphocytes and the influence of coactivating molecules on these signaling processes is not fully understood. To better understand the role of a key second messenger, calcium, in governing thymocyte maturation, we measured the intracellular free calcium concentration ([Ca2+]i) response to changes in TCR avidity and costimulation. We found that TCR stimulation initiates either amplitude- or frequency-encoded [Ca2+]i changes depending on (a) the maturation state of stimulated thymocytes, (b) the avidity of TCR interactions, and (c) the participation of specific coactivating molecules. Calcium signaling within immature but not mature thymocytes could be modulated by the avidity of CD3/CD4 engagement. Low avidity interactions induced biphasic calcium responses, whereas high avidity engagement initiated oscillatory calcium changes. Notably, CD28 participation converted the calcium response to low avidity receptor engagement from a biphasic to oscillatory pattern. These data suggest that calcium plays a central role in encoding the nature of the TCR signal received by thymocytes and, consequently, a role in thymic selection.
Figures







Similar articles
-
Maturation versus death of developing double-positive thymocytes reflects competing effects on Bcl-2 expression and can be regulated by the intensity of CD28 costimulation.J Immunol. 2001 Mar 1;166(5):3468-75. doi: 10.4049/jimmunol.166.5.3468. J Immunol. 2001. PMID: 11207305
-
Regulation of thymocyte development through CD3. I. Timepoint of ligation of CD3 epsilon determines clonal deletion or induction of developmental program.J Exp Med. 1993 Mar 1;177(3):707-16. doi: 10.1084/jem.177.3.707. J Exp Med. 1993. PMID: 8382254 Free PMC article.
-
CD4 and CD8 are positive regulators of T cell receptor signal transduction in early T cell differentiation.J Immunol. 1991 Mar 15;146(6):1759-65. J Immunol. 1991. PMID: 1706380
-
Cross-talk between the T cell antigen receptor and the glucocorticoid receptor regulates thymocyte development.Stem Cells. 1996 Sep;14(5):490-500. doi: 10.1002/stem.140490. Stem Cells. 1996. PMID: 8888490 Review.
-
Positive and negative thymocyte selection.Crit Rev Immunol. 1998;18(4):359-70. doi: 10.1615/critrevimmunol.v18.i4.40. Crit Rev Immunol. 1998. PMID: 9704194 Review.
Cited by
-
Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction.Proc Natl Acad Sci U S A. 2005 Nov 22;102(47):17071-6. doi: 10.1073/pnas.0506070102. Epub 2005 Nov 14. Proc Natl Acad Sci U S A. 2005. PMID: 16286658 Free PMC article.
-
ORAI1 deficiency impairs activated T cell death and enhances T cell survival.J Immunol. 2011 Oct 1;187(7):3620-30. doi: 10.4049/jimmunol.1100847. Epub 2011 Aug 26. J Immunol. 2011. PMID: 21873530 Free PMC article.
-
Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling.Nat Immunol. 2012 May 6;13(6):560-8. doi: 10.1038/ni.2301. Nat Immunol. 2012. PMID: 22561606
-
STIM- and Orai-mediated calcium entry controls NF-κB activity and function in lymphocytes.Cell Calcium. 2018 Sep;74:131-143. doi: 10.1016/j.ceca.2018.07.003. Epub 2018 Jul 10. Cell Calcium. 2018. PMID: 30048879 Free PMC article. Review.
-
Protein kinase Calpha (PKCalpha) acts upstream of PKCtheta to activate IkappaB kinase and NF-kappaB in T lymphocytes.Mol Cell Biol. 2003 Oct;23(19):7068-81. doi: 10.1128/MCB.23.19.7068-7081.2003. Mol Cell Biol. 2003. PMID: 12972622 Free PMC article.
References
-
- Ashton-Rickardt P.G., Bandeira A., Delaney J.R., Van Kaer L., Pircher H.P., Zinkernagel R.M., Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. - PubMed
-
- Teh H.S., Motyka B., Teh S.J. Influence of the affinity of selecting ligands on T cell positive and negative selection and the functional maturity of the positively selected T cells. Crit. Rev. Immunol. 1997;17:399–410. - PubMed
-
- Chidgey A.P., Boyd R.L. Positive selection of low responsive, potentially autoreactive T cells induced by high avidity, non-deleting interactions. Int. Immunol. 1998;10:999–1008. - PubMed
-
- Ashton-Rickardt P.G., Tonegawa S. A differential-avidity model for T-cell selection. Immunol. Today. 1994;15:362–366. - PubMed
-
- Cibotti R., Punt J.A., Dash K.S., Sharrow S.O., Singer A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity. 1997;6:245–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

