Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog
- PMID: 10510326
- PMCID: PMC408560
- DOI: 10.1172/JCI7691
Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog
Abstract
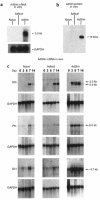
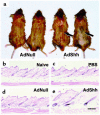
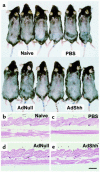
Hair follicles form in prenatal skin and mature in the postnatal period, establishing a growth cycle in 3 phases: telogen (resting), anagen (growth), and catagen (regression). Based on the knowledge that Sonic hedgehog (Shh) expression is necessary for the embryonic development of hair follicles, and that anagen in the postnatal cycling follicle has morphologic similarities to the epithelial invagination process in embryonic skin, we hypothesized that localized, but transient, enhanced expression of the Shh gene in postnatal skin would accelerate initiation of anagen in the hair follicle cycle, with concomitant accelerated hair growth. To assess this concept, an E1(-) adenovirus vector, AdShh, was used to transfer the murine Shh cDNA to skin of postnatal day 19 C57BL/6 mice. The treated skin showed increased mRNA expression of Shh, Patched (the Shh receptor), and Gli1 (a transcription factor in the Shh pathway). In mice receiving AdShh, but not in controls, acceleration into anagen was evident, since hair follicle size and melanogenesis increased and the hair-specific keratin ghHb-1 and the melanin synthesis-related tyrosinase mRNAs accumulated. Finally, C57BL/6 mice showed marked acceleration of the onset of new hair growth in the region of AdShh administration to skin 2 weeks after treatment, but not in control vector-treated or untreated areas. After 6 months, AdShh-treated skin showed normal hair and normal skin morphology. Together, these observations are consistent with the concept that upregulation of Shh activity in postnatal skin functions as a biologic switch that induces resting hair follicles to enter anagen with consequent hair growth.
Figures



Comment in
-
The Hedgehog and the hair follicle: a growing relationship.J Clin Invest. 1999 Oct;104(7):851-3. doi: 10.1172/JCI8416. J Clin Invest. 1999. PMID: 10510325 Free PMC article. Review. No abstract available.
Similar articles
-
Effect of adenovirus-mediated expression of Sonic hedgehog gene on hair regrowth in mice with chemotherapy-induced alopecia.J Natl Cancer Inst. 2001 Dec 19;93(24):1858-64. doi: 10.1093/jnci/93.24.1858. J Natl Cancer Inst. 2001. PMID: 11752010
-
In vivo enhanced expression of patched dampens the sonic hedgehog pathway.Mol Ther. 2002 Aug;6(2):258-64. doi: 10.1006/mthe.2002.0628. Mol Ther. 2002. PMID: 12161193
-
Noggin is required for induction of the hair follicle growth phase in postnatal skin.FASEB J. 2001 Oct;15(12):2205-14. doi: 10.1096/fj.01-0207com. FASEB J. 2001. PMID: 11641247
-
STAT5 Activation in the Dermal Papilla Is Important for Hair Follicle Growth Phase Induction.J Invest Dermatol. 2016 Sep;136(9):1781-1791. doi: 10.1016/j.jid.2016.04.014. Epub 2016 Apr 27. J Invest Dermatol. 2016. PMID: 27131881 Review.
-
A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages.J Invest Dermatol. 2001 Jul;117(1):3-15. doi: 10.1046/j.0022-202x.2001.01377.x. J Invest Dermatol. 2001. PMID: 11442744 Review.
Cited by
-
Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats.Animals (Basel). 2024 Jul 12;14(14):2049. doi: 10.3390/ani14142049. Animals (Basel). 2024. PMID: 39061511 Free PMC article.
-
The Hedgehog and the hair follicle: a growing relationship.J Clin Invest. 1999 Oct;104(7):851-3. doi: 10.1172/JCI8416. J Clin Invest. 1999. PMID: 10510325 Free PMC article. Review. No abstract available.
-
Partial proteasome inhibitors induce hair follicle growth by stabilizing β-catenin.Stem Cells. 2014 Jan;32(1):85-92. doi: 10.1002/stem.1525. Stem Cells. 2014. PMID: 23963711 Free PMC article.
-
A desmosomal cadherin controls multipotent hair follicle stem cell quiescence and orchestrates regeneration through adhesion signaling.iScience. 2023 Nov 25;26(12):108568. doi: 10.1016/j.isci.2023.108568. eCollection 2023 Dec 15. iScience. 2023. PMID: 38162019 Free PMC article.
-
Wnt signaling maintains the hair-inducing activity of the dermal papilla.Genes Dev. 2000 May 15;14(10):1181-5. Genes Dev. 2000. PMID: 10817753 Free PMC article.
References
-
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. - PubMed
-
- Oro AE, Scott MP. Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell. 1998;95:575–578. - PubMed
-
- Sengel, P. 1976. Morphogenesis of skin. Cambridge University Press. Cambridge, United Kingdom. pp. 20–29.
-
- Wessells NK, Rommens JM. Nonproliferation in dermal condensations of mouse vibrissae and pelage hairs. Dev Biol. 1965;12:419–433. - PubMed
-
- Bertolino, A.P., Klein, L.M., and Freedberg, I.M. 1993. Biology of hair follicles. In Dermatology in general medicine. T.B. Fitzpatrick, A.Z. Eisen, K. Wolff, I.M. Freedberg, and K.F. Austen, editors. McGraw-Hill Inc. New York, NY. 289–293.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

