Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance
- PMID: 10515900
- PMCID: PMC88922
- DOI: 10.1128/CMR.12.4.501
Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance
Abstract
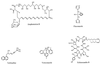
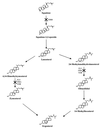
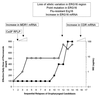
The increased use of antibacterial and antifungal agents in recent years has resulted in the development of resistance to these drugs. The significant clinical implication of resistance has led to heightened interest in the study of antimicrobial resistance from different angles. Areas addressed include mechanisms underlying this resistance, improved methods to detect resistance when it occurs, alternate options for the treatment of infections caused by resistant organisms, and strategies to prevent and control the emergence and spread of resistance. In this review, the mode of action of antifungals and their mechanisms of resistance are discussed. Additionally, an attempt is made to discuss the correlation between fungal and bacterial resistance. Antifungals can be grouped into three classes based on their site of action: azoles, which inhibit the synthesis of ergosterol (the main fungal sterol); polyenes, which interact with fungal membrane sterols physicochemically; and 5-fluorocytosine, which inhibits macromolecular synthesis. Many different types of mechanisms contribute to the development of resistance to antifungals. These mechanisms include alteration in drug target, alteration in sterol biosynthesis, reduction in the intercellular concentration of target enzyme, and overexpression of the antifungal drug target. Although the comparison between the mechanisms of resistance to antifungals and antibacterials is necessarily limited by several factors defined in the review, a correlation between the two exists. For example, modification of enzymes which serve as targets for antimicrobial action and the involvement of membrane pumps in the extrusion of drugs are well characterized in both the eukaryotic and prokaryotic cells.
Figures
Comment in
-
Quality control parameters for broth microdilution tests of anidulafungin.J Clin Microbiol. 2004 Jan;42(1):490. doi: 10.1128/JCM.42.1.490.2004. J Clin Microbiol. 2004. PMID: 14715814 Free PMC article. No abstract available.
References
-
- Anaissie E J, Bodey G P. Nosocomial fungal infections—old problems and new challenges. Infect Dis Clin North Am. 1989;3:867–882. - PubMed
-
- Arthur M, Reynolds P, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol. 1996;4:401–407. - PubMed
-
- Ather M A, Winner H I. Development of resistance by Candida species to polyene antibiotics in vitro. J Med Microbiol. 1971;4:505–517. - PubMed
-
- Beck-Sagué C M, Jarvis W R National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

