Methods for subtyping and molecular comparison of human viral genomes
- PMID: 10515905
- PMCID: PMC88927
- DOI: 10.1128/CMR.12.4.612
Methods for subtyping and molecular comparison of human viral genomes
Abstract
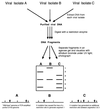
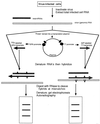
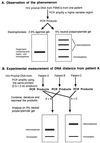
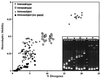
The development over the past two decades of molecular methods for manipulation of RNA and DNA has afforded molecular virologists the ability to study viral genomes in detail that has heretofore not been possible. There are many molecular techniques now available for typing and subtyping of viruses. The available methods include restriction fragment length polymorphism analysis, Southern blot analysis, oligonucleotide fingerprint analysis, reverse hybridization, DNA enzyme immunoassay, RNase protection analysis, single-strand conformation polymorphism analysis, heteroduplex mobility assay, nucleotide sequencing, and genome segment length polymorphism analysis. The methods have certain advantages and disadvantages which should be considered in their application to specific viruses or for specific purposes. These techniques are likely to become more widely used in the future for epidemiologic studies and for investigations into the pathophysiology of virus infections.
Figures



References
-
- Adams S G, Dohner D E, Gelb L D. Restriction fragment differences between the genomes of the Oka varicella vaccine virus and American wild-type varicella-zoster virus. J Med Virol. 1989;29:38–45. - PubMed
-
- Agostini H T, Ryschkewitsch C F, Mory R, Singer E J, Stoner G L. JC virus (JCV) genotypes in brain tissue from patients with progressive multifocal leukoencephalopathy (PML) and in urine from controls without PML: increased frequency of JCV type 2 in PML. J Infect Dis. 1997;176:1–8. - PubMed
-
- Ando T, Jin Q, Gentsch J R, Monroe S S, Noel J S, Dowell S F, Cicirello H G, Kohn M A, Glass R I. Epidemiologic applications of novel molecular methods to detect and differentiate small round structured viruses (Norwalk-like viruses) J Med Virol. 1995;47:145–152. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

