Purification of P(II) and P(II)-UMP and in vitro studies of regulation of glutamine synthetase in Rhodospirillum rubrum
- PMID: 10515945
- PMCID: PMC103790
- DOI: 10.1128/JB.181.20.6524-6529.1999
Purification of P(II) and P(II)-UMP and in vitro studies of regulation of glutamine synthetase in Rhodospirillum rubrum
Abstract
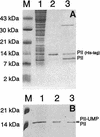
The P(II) protein from Rhodospirillum rubrum was fused with a histidine tag, overexpressed in Escherichia coli, and purified by Ni(2+)-chelating chromatography. The uridylylated form of the P(II) protein could be generated in E. coli. The effects on the regulation of glutamine synthetase by P(II), P(II)-UMP, glutamine, and alpha-ketoglutarate were studied in extracts from R. rubrum grown under different conditions. P(II) and glutamine were shown to stimulate the ATP-dependent inactivation (adenylylation) of glutamine synthetase, which could be totally inhibited by alpha-ketoglutarate. Deadenylylation (activation) of glutamine synthetase required phosphate, but none of the effectors studied had any major effect, which is different from their role in the E. coli system. In addition, deadenylylation was found to be much slower than adenylylation under the conditions investigated.
Figures
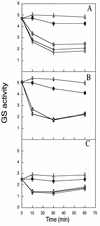
 , 10 mM glutamine and incubated at 30°C. Extracts are from an N-starved culture (A), an N2-grown culture (B), and a switch-off culture (C). The experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.
, 10 mM glutamine and incubated at 30°C. Extracts are from an N-starved culture (A), an N2-grown culture (B), and a switch-off culture (C). The experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.
 , 0.2 mM; □, 0.5 mM; ▵, 1 mM;
, 0.2 mM; □, 0.5 mM; ▵, 1 mM;  , 2.5 mM; ▾, 5 mM. Extracts were incubated at 30°C. The experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.
, 2.5 mM; ▾, 5 mM. Extracts were incubated at 30°C. The experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.
 , 0.09 μM; ■, 0.17 μM; ▴, 0.43 μM;
, 0.09 μM; ■, 0.17 μM; ▴, 0.43 μM;  , 0.85 μM; □, 1.31 μM; ▵, 1.97 μM; ◊, 2.56 μM. The boxed insert shows glutamine synthetase activity after 10 min at different PII concentrations. (B) Extract from N-starved cells with 2 mM α-ketoglutarate and glutamine (final concentrations as follows). ⊞, no addition;
, 0.85 μM; □, 1.31 μM; ▵, 1.97 μM; ◊, 2.56 μM. The boxed insert shows glutamine synthetase activity after 10 min at different PII concentrations. (B) Extract from N-starved cells with 2 mM α-ketoglutarate and glutamine (final concentrations as follows). ⊞, no addition;  , 1 mM; ■, 5 mM; ▴, 10 mM;
, 1 mM; ■, 5 mM; ▴, 10 mM;  , 20 mM. The boxed insert shows glutamine synthetase activity after 10 min at different glutamine concentrations. Experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.
, 20 mM. The boxed insert shows glutamine synthetase activity after 10 min at different glutamine concentrations. Experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.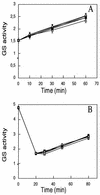
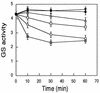
 , 0.85 μM PII-UMP; ◊, 10 mM glutamine. (B) Extract from an N2-grown culture supplemented by 2 mM ATP and 6 mM MgCl2. After 20 min, the extract was divided into tubes containing 10 mM Pi and other effectors as in (A). The experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.
, 0.85 μM PII-UMP; ◊, 10 mM glutamine. (B) Extract from an N2-grown culture supplemented by 2 mM ATP and 6 mM MgCl2. After 20 min, the extract was divided into tubes containing 10 mM Pi and other effectors as in (A). The experiments were carried out several times. The data are from one representative experiment with duplicate samples. Glutamine synthetase activity is given in micromoles of γ-glutamyl hydroxamate/minute · milligrams of protein.Similar articles
-
In vitro studies of the uridylylation of the three PII protein paralogs from Rhodospirillum rubrum: the transferase activity of R. rubrum GlnD is regulated by alpha-ketoglutarate and divalent cations but not by glutamine.J Bacteriol. 2007 May;189(9):3471-8. doi: 10.1128/JB.01704-06. Epub 2007 Mar 2. J Bacteriol. 2007. PMID: 17337583 Free PMC article.
-
The activity of adenylyltransferase in Rhodospirillum rubrum is only affected by alpha-ketoglutarate and unmodified PII proteins, but not by glutamine, in vitro.FEBS J. 2007 May;274(10):2449-60. doi: 10.1111/j.1742-4658.2007.05778.x. Epub 2007 Apr 5. FEBS J. 2007. PMID: 17419734
-
The regulation of Escherichia coli glutamine synthetase revisited: role of 2-ketoglutarate in the regulation of glutamine synthetase adenylylation state.Biochemistry. 1998 Sep 15;37(37):12802-10. doi: 10.1021/bi980666u. Biochemistry. 1998. PMID: 9737857
-
Escherichia coli glutamine synthetase adenylyltransferase (ATase, EC 2.7.7.49): kinetic characterization of regulation by PII, PII-UMP, glutamine, and alpha-ketoglutarate.Biochemistry. 2007 Apr 3;46(13):4133-46. doi: 10.1021/bi0620510. Epub 2007 Mar 14. Biochemistry. 2007. PMID: 17355125
-
H2 metabolism in photosynthetic bacteria and relationship to N2 fixation.Ann Microbiol (Paris). 1983 Jul-Aug;134B(1):115-35. doi: 10.1016/s0769-2609(83)80100-8. Ann Microbiol (Paris). 1983. PMID: 6139053 Review.
Cited by
-
Molecular basis for the distinct divalent cation requirement in the uridylylation of the signal transduction proteins GlnJ and GlnB from Rhodospirillum rubrum.BMC Microbiol. 2012 Jul 8;12:136. doi: 10.1186/1471-2180-12-136. BMC Microbiol. 2012. PMID: 22769741 Free PMC article.
-
In vitro studies of the uridylylation of the three PII protein paralogs from Rhodospirillum rubrum: the transferase activity of R. rubrum GlnD is regulated by alpha-ketoglutarate and divalent cations but not by glutamine.J Bacteriol. 2007 May;189(9):3471-8. doi: 10.1128/JB.01704-06. Epub 2007 Mar 2. J Bacteriol. 2007. PMID: 17337583 Free PMC article.
-
GlnD is essential for NifA activation, NtrB/NtrC-regulated gene expression, and posttranslational regulation of nitrogenase activity in the photosynthetic, nitrogen-fixing bacterium Rhodospirillum rubrum.J Bacteriol. 2005 Feb;187(4):1254-65. doi: 10.1128/JB.187.4.1254-1265.2005. J Bacteriol. 2005. PMID: 15687189 Free PMC article.
-
P(II) signal transduction proteins, pivotal players in microbial nitrogen control.Microbiol Mol Biol Rev. 2001 Mar;65(1):80-105. doi: 10.1128/MMBR.65.1.80-105.2001. Microbiol Mol Biol Rev. 2001. PMID: 11238986 Free PMC article. Review.
-
Yeast two-hybrid studies on interaction of proteins involved in regulation of nitrogen fixation in the phototrophic bacterium Rhodobacter capsulatus.J Bacteriol. 2003 Sep;185(17):5240-7. doi: 10.1128/JB.185.17.5240-5247.2003. J Bacteriol. 2003. PMID: 12923097 Free PMC article.
References
-
- Atkinson M R, Ninfa A J. Role of the GlnK signal transduction protein in the regulation of nitrogen assimilation in Escherichia coli. Mol Microbiol. 1998;29:431–447. - PubMed
-
- de Zamaroczy M. Structural homologues PII and PZ of Azospirillum brasilense provide intracellular signalling for selective regulation of various nitrogen-dependent functions. Mol Microbiol. 1998;29:449–463. - PubMed
-
- Falk G, Johansson B C, Nordlund S. The role of glutamine synthetase in the regulation of nitrogenase activity (switch-off effect) in Rhodospirillum rubrum. Arch Microbiol. 1982;132:251–253.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

