Mutant cells selected during persistent reovirus infection do not express mature cathepsin L and do not support reovirus disassembly
- PMID: 10516062
- PMCID: PMC112988
- DOI: 10.1128/JVI.73.11.9532-9543.1999
Mutant cells selected during persistent reovirus infection do not express mature cathepsin L and do not support reovirus disassembly
Abstract
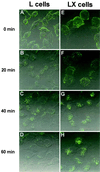
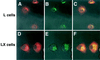
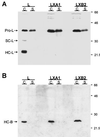
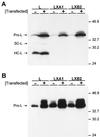
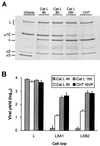
Persistent reovirus infections of murine L929 cells select cellular mutations that inhibit viral disassembly within the endocytic pathway. Mutant cells support reovirus growth when infection is initiated with infectious subvirion particles (ISVPs), which are intermediates in reovirus disassembly formed following proteolysis of viral outer-capsid proteins. However, mutant cells do not support growth of virions, indicating that these cells have a defect in virion-to-ISVP processing. To better understand mechanisms by which viruses use the endocytic pathway to enter cells, we defined steps in reovirus replication blocked in mutant cells selected during persistent infection. Subcellular localization of reovirus after adsorption to parental and mutant cells was assessed using confocal microscopy and virions conjugated to a fluorescent probe. Parental and mutant cells did not differ in the capacity to internalize virions or distribute them to perinuclear compartments. Using pH-sensitive probes, the intravesicular pH was determined and found to be equivalent in parental and mutant cells. In both cell types, virions localized to acidified intracellular organelles. The capacity of parental and mutant cells to support proteolysis of reovirus virions was assessed by monitoring the appearance of disassembly intermediates following adsorption of radiolabeled viral particles. Within 2 h after adsorption to parental cells, proteolysis of viral outer-capsid proteins was observed, consistent with formation of ISVPs. However, in mutant cells, no proteolysis of viral proteins was detected up to 8 h postadsorption. Since treatment of cells with E64, an inhibitor of cysteine-containing proteases, blocks reovirus disassembly, we used immunoblot analysis to assess the expression of cathepsin L, a lysosomal cysteine protease. In contrast to parental cells, mutant cells did not express the mature, proteolytically active form of the enzyme. The defect in cathepsin L maturation was not associated with mutations in procathepsin L mRNA, was not complemented by procathepsin L overexpression, and did not affect the maturation of cathepsin B, another lysosomal cysteine protease. These findings indicate that persistent reovirus infections select cellular mutations that affect the maturation of cathepsin L and suggest that alterations in the expression of lysosomal proteases can modulate viral cytopathicity.
Figures



References
-
- Ahmed R, Canning W M, Kauffman R S, Sharpe A H, Hallum J V, Fields B N. Role of the host cell in persistent viral infection: coevolution of L cells and reovirus during persistent infection. Cell. 1981;25:325–332. - PubMed
-
- Ahmed R, Fields B N. Role of the S4 gene in the establishment of persistent reovirus infection in L cells. Cell. 1982;28:605–612. - PubMed
-
- Ahmed R, Kauffman R S, Fields B N. Genetic variation during persistent reovirus infection: isolation of cold-sensitive and temperature-sensitive mutants from persistently infected L cells. Virology. 1983;131:71–78. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

