Identification of a cis-acting dendritic targeting element in MAP2 mRNAs
- PMID: 10516301
- PMCID: PMC6782761
- DOI: 10.1523/JNEUROSCI.19-20-08818.1999
Identification of a cis-acting dendritic targeting element in MAP2 mRNAs
Abstract
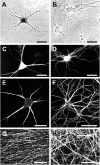
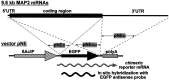
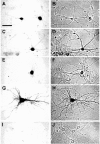
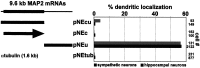
In neurons, a limited number of mRNAs have been identified in dendritic processes, whereas other transcripts are restricted to the cell soma. Here we have investigated the molecular mechanisms underlying extrasomatic localization of mRNAs encoding microtubule-associated protein 2 (MAP2) in primary neuronal cultures. Vectors expressing recombinant mRNAs were introduced into hippocampal and sympathetic neurons using DNA transfection and microinjection protocols, respectively. Chimeric mRNAs containing the entire 3' untranslated region of MAP2 transcripts fused to a nondendritic reporter mRNA are detected in dendrites. In contrast, RNAs containing MAP2 coding and 5' untranslated regions or tubulin sequences are restricted to the cell soma. Moreover, 640 nucleotides from the MAP2 3' untranslated region (UTR) are both sufficient and essential for extrasomatic localization of chimeric mRNAs in hippocampal and sympathetic neurons. Thus, a cis-acting dendritic targeting element that is effective in two distinct neuronal cell types is contained in the 3' UTR of MAP2 transcripts. The observation of RNA granules in dendrites implies that extrasomatic transcripts seem to assemble into multimolecular complexes that may function as transport units.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
