Evidence of presynaptic location and function of the prion protein
- PMID: 10516306
- PMCID: PMC6782778
- DOI: 10.1523/JNEUROSCI.19-20-08866.1999
Evidence of presynaptic location and function of the prion protein
Abstract
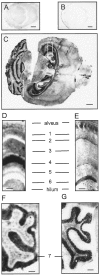
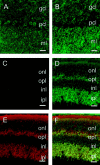
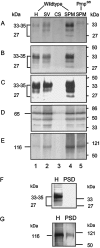
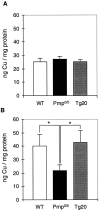
The prion protein (PrP(C)) is a copper-binding protein of unknown function that plays an important role in the etiology of transmissible spongiform encephalopathies. Using morphological techniques and synaptosomal fractionation methods, we show that PrP(C) is predominantly localized to synaptic membranes. Atomic absorption spectroscopy was used to identify PrP(C)-related changes in the synaptosomal copper concentration in transgenic mouse lines. The synaptic transmission in the presence of H(2)O(2), which is known to be decomposed to highly reactive hydroxyl radicals in the presence of iron or copper and to alter synaptic activity, was studied in these animals. The response of synaptic activity to H(2)O(2) was found to correlate with the amount of PrP(C) expression in the presynaptic neuron in cerebellar slice preparations from wild-type, Prnp(0/0), and PrP gene-reconstituted transgenic mice. Thus, our data gives strong evidence for the predominantly synaptic location of PrP(C), its involvement in the regulation of the presynaptic copper concentration, and synaptic activity in defined conditions.
Figures


Similar articles
-
Molecular genetics of transmissible spongiform encephalopathies: an introduction.J Toxicol Sci. 2002 May;27(2):69-77. doi: 10.2131/jts.27.69. J Toxicol Sci. 2002. PMID: 12058449 Review.
-
Genetic mapping of activity determinants within cellular prion proteins: N-terminal modules in PrPC offset pro-apoptotic activity of the Doppel helix B/B' region.J Biol Chem. 2004 Dec 31;279(53):55443-54. doi: 10.1074/jbc.M404794200. Epub 2004 Sep 29. J Biol Chem. 2004. PMID: 15459186
-
Prion protein and neuronal differentiation: quantitative analysis of prnp gene expression in a murine inducible neuroectodermal progenitor.Microbes Infect. 1999 Oct;1(12):969-76. doi: 10.1016/s1286-4579(99)80514-0. Microbes Infect. 1999. PMID: 10617928
-
Neuroprotective role of PrPC against kainate-induced epileptic seizures and cell death depends on the modulation of JNK3 activation by GluR6/7-PSD-95 binding.Mol Biol Cell. 2011 Sep;22(17):3041-54. doi: 10.1091/mbc.E11-04-0321. Epub 2011 Jul 14. Mol Biol Cell. 2011. PMID: 21757544 Free PMC article.
-
The prion gene complex encoding PrP(C) and Doppel: insights from mutational analysis.Gene. 2001 Sep 5;275(1):1-18. doi: 10.1016/s0378-1119(01)00627-8. Gene. 2001. PMID: 11574147 Review.
Cited by
-
Octarepeat region flexibility impacts prion function, endoproteolysis and disease manifestation.EMBO Mol Med. 2015 Mar;7(3):339-56. doi: 10.15252/emmm.201404588. EMBO Mol Med. 2015. PMID: 25661904 Free PMC article.
-
Perturbed signal transduction in neurodegenerative disorders involving aberrant protein aggregation.Neuromolecular Med. 2003;4(1-2):109-32. doi: 10.1385/NMM:4:1-2:109. Neuromolecular Med. 2003. PMID: 14528056 Review.
-
Insoluble cellular prion protein and its association with prion and Alzheimer diseases.Prion. 2011 Jul-Sep;5(3):172-8. doi: 10.4161/pri.5.3.16894. Epub 2011 Jul 1. Prion. 2011. PMID: 21847014 Free PMC article. Review.
-
Diminished Neuronal ESCRT-0 Function Exacerbates AMPA Receptor Derangement and Accelerates Prion-Induced Neurodegeneration.J Neurosci. 2023 May 24;43(21):3970-3984. doi: 10.1523/JNEUROSCI.1878-22.2023. Epub 2023 Apr 5. J Neurosci. 2023. PMID: 37019623 Free PMC article.
-
The octarepeat domain of the prion protein binds Cu(II) with three distinct coordination modes at pH 7.4.J Am Chem Soc. 2005 Sep 14;127(36):12647-56. doi: 10.1021/ja053254z. J Am Chem Soc. 2005. PMID: 16144413 Free PMC article.
References
-
- Bendheim PE, Brown HR, Rudelli RD, Scala LJ, Goller NL, Wen GY, Kascsak RJ, Cashman NR, Bolton DC. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology. 1992;42:149–156. - PubMed
-
- Brose N, Halpain S, Suchanek C, Jahn R. Characterization and partial purification of a chloride- and calcium-dependent glutamate-binding protein from rat brain. J Biol Chem. 1989;264:9619–9625. - PubMed
-
- Brose N, Huntley GW, Stern-Bach Y, Sharma G, Morrison JH, Heinemann SF. Differential assembly of coexpressed glutamate receptor subunits in neurons of rat cerebral cortex. J Biol Chem. 1994;269:16780–16784. - PubMed
-
- Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser P, Kruck T, von Bohlen A, Schulz-Schaeffer W, Giese A, Westaway D, Kretzschmar H. The cellular prion protein binds copper in vivo. Nature. 1997a;390:684–687. - PubMed
-
- Brown DR, Schulz-Schaeffer WJ, Schmidt B, Kretzschmar HA. Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp Neurol. 1997b;146:104–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
