Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch
- PMID: 10516328
- PMCID: PMC6782742
- DOI: 10.1523/JNEUROSCI.19-20-09107.1999
Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch
Abstract
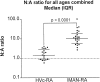
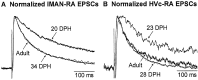
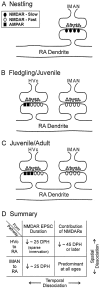
In most songbirds, vocal learning occurs through two experience-dependent phases, culminating in a reduction of behavioral plasticity called song crystallization. At ends of developmentally plastic periods in other systems, synaptic properties change in a fashion appropriate to limit plasticity. Maturation of glutamatergic synapses often involves a reduction in duration of NMDA receptor (NMDAR)-mediated synaptic responses and a coincident reduction in the contribution of NMDARs to synaptic transmission. We hypothesized that similar changes in the zebra finch song system help limit behavioral plasticity during song development. Nucleus robustus archistriatalis (RA) is a key nucleus in the forebrain song motor pathway and receives glutamatergic input from the motor nucleus HVc. RA also receives glutamatergic input, mediated primarily by NMDARs, from the lateral magnocellular nucleus of the anterior neostriatum, which is part of a circuit essential for learning but not song production. We examined whether synaptic maturation occurs in either input to RA by recording synaptic currents in brain slices prepared from zebra finches of different ages. We find the motor input from HVc to RA uses both AMPA receptors (AMPARs) and NMDARs, and synaptic maturation occurs in two phases: an early reduction in duration of NMDAR-mediated synaptic currents in both inputs, and a later reduction in the NMDAR contribution to synaptic responses in the motor pathway. Although NMDAR kinetics change too early to account for crystallization, the reduction of the relative NMDAR contribution to synaptic transmission could contribute to the onset of crystallization. Thus, synaptic maturation events can be temporally distinct and input-specific and may play different roles in behavioral plasticity.
Figures





References
-
- Aamodt SM, Nordeen EJ, Nordeen KW. Early isolation from conspecific song does not affect the normal developmental decline of N-methyl-d-aspartate receptor binding in an avian song nucleus. J Neurobiol. 1994;27:76–84. - PubMed
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Arnold AP. The effects of castration on song development in zebra finches (Poephila guttata). J Exp Zool. 1975;191:261–278. - PubMed
-
- Basham ME, Nordeen EJ, Nordeen KW. Developmental regulation of NMDA2B receptor binding in the zebra finch anterior forebrain. Soc Neurosci Abstr. 1997;23:797.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
