Direct actions of nitric oxide on rat neurohypophysial K+ channels
- PMID: 10517809
- PMCID: PMC2269563
- DOI: 10.1111/j.1469-7793.1999.00165.x
Direct actions of nitric oxide on rat neurohypophysial K+ channels
Abstract
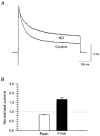
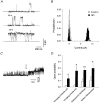
1. Nitric oxide (NO) has been shown to modulate neuropeptide secretion from the posterior pituitary. Here we show that NO activates large-conductance Ca2+-activated K+ (BK) channels in posterior pituitary nerve terminals. 2. NO, generated either by the photolysis of caged-NO or with chemical donors, irreversibly enhanced the component of whole-terminal K+ current due to BK channels and increased the activity of BK channels in excised patches. NO also inhibited the transient A-current. The time courses of these effects on K+ current were very different; activation of BK channels developed slowly over several minutes whereas inhibition of A-current immediately followed NO uncaging. 3. Activation of BK channels by NO occurred in the presence of guanylyl cyclase inhibitors and after removal of ATP or GTP from the pipette solution, suggesting a cGMP-independent signalling pathway. 4. The sulfhydryl alkylating agent N-ethyl maleimide (NEM) increased BK channel activity. Pretreatment with NEM occluded NO activation. 5. NO activation of BK channels occurred independently of voltage and cytoplasmic Ca2+ concentration. In addition, NO removed the strict Ca2+ requirement for channel activation, rendering channels highly active even at nanomolar Ca2+ levels. 6. These results suggest that NO, or a reactive nitrogen byproduct, chemically modifies nerve terminal BK channels or a closely associated protein and thereby produces an increase in channel activity. Such activation is likely to inhibit impulse activity in posterior pituitary nerve terminals and this may explain the inhibitory action of NO on secretion.
Figures







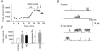
References
-
- Arnelle DR, Stamler JS. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Archives of Biochemistry and Biophysics. 1995;318:279–285. - PubMed
-
- Bettache N, Carter T, Corrie JE, Ogden D, Trentham DR. Photolabile donors of nitric oxide: ruthenium nitrosyl chlorides as caged nitric oxide. Methods in Enzymology. 1996;268:266–281. - PubMed
-
- Bielefeldt K, Jackson MB. A calcium-activated potassium channel causes frequency-dependent action-potential failures in a mammalian nerve terminal. Journal of Neurophysiology. 1993;70:284–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

