The role of Ca2+ feedback in shaping InsP3-evoked Ca2+ signals in mouse pancreatic acinar cells
- PMID: 10517811
- PMCID: PMC2269552
- DOI: 10.1111/j.1469-7793.1999.00187.x
The role of Ca2+ feedback in shaping InsP3-evoked Ca2+ signals in mouse pancreatic acinar cells
Abstract
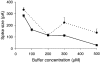
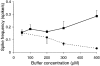
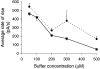
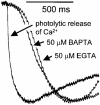
1. Cytosolic Ca2+ has been proposed to act as both a positive and a negative feedback signal on the inositol trisphosphate (InsP3) receptor. However, it is unclear how this might affect the Ca2+ response in vivo. 2. Mouse pancreatic acinar cells were whole-cell patch clamped to record the Ca2+-dependent chloride (Cl(Ca)) current spikes and imaged to record the cytosolic Ca2+ spikes elicited by the injection of Ins(2,4,5)P3. Increasing concentrations of Ca2+ buffer (up to 200 microM EGTA or BAPTA) were associated with the appearance of steps in the current activation phase and a prevalence of smaller-amplitude Cl(Ca) spikes. Imaging experiments showed that with increased buffer the secretory pole cytosolic Ca2+ signal became fragmented and spatially discrete Ca2+ release events were observed. 3. At higher buffer concentrations (200-500 microM), increasing concentrations of EGTA increased spike frequency and reduced spike amplitude. In contrast, BAPTA decreased spike frequency and maintained large spike amplitudes. 4. We conclude that, during InsP3-evoked spiking, long-range Ca2+ feedback ( approximately 2-4 microm) shapes the rising phase of the Ca2+ signal by acting to co-ordinate discrete Ca2+ release events and short-range ( approximately 40 nm) Ca2+ feedback acts to inhibit further Ca2+ release.
Figures


Similar articles
-
Kinetics of Ca2+ release by InsP3 in pig single aortic endothelial cells: evidence for an inhibitory role of cytosolic Ca2+ in regulating hormonally evoked Ca2+ spikes.J Physiol. 1997 Oct 1;504 ( Pt 1)(Pt 1):17-33. doi: 10.1111/j.1469-7793.1997.00017.x. J Physiol. 1997. PMID: 9350614 Free PMC article.
-
Mechanisms underlying InsP3-evoked global Ca2+ signals in mouse pancreatic acinar cells.J Physiol. 2000 Aug 1;526 Pt 3(Pt 3):515-26. doi: 10.1111/j.1469-7793.2000.t01-1-00515.x. J Physiol. 2000. PMID: 10922004 Free PMC article.
-
Ca2+ influx modulation of temporal and spatial patterns of inositol trisphosphate-mediated Ca2+ liberation in Xenopus oocytes.J Physiol. 1994 Apr 1;476(1):17-28. J Physiol. 1994. PMID: 8046631 Free PMC article.
-
Local calcium spiking in pancreatic acinar cells.Ciba Found Symp. 1995;188:85-94; discussion 94-103. Ciba Found Symp. 1995. PMID: 7587625 Review.
-
Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications.Cell Calcium. 1993 Nov;14(10):746-57. doi: 10.1016/0143-4160(93)90100-k. Cell Calcium. 1993. PMID: 8131191 Review.
Cited by
-
Calcium wave propagation in pancreatic acinar cells: functional interaction of inositol 1,4,5-trisphosphate receptors, ryanodine receptors, and mitochondria.J Gen Physiol. 2000 Oct;116(4):547-60. doi: 10.1085/jgp.116.4.547. J Gen Physiol. 2000. PMID: 11004204 Free PMC article.
-
Cytosolic Ca(2+) and Ca(2+)-activated Cl(-) current dynamics: insights from two functionally distinct mouse exocrine cells.J Physiol. 2002 Apr 15;540(Pt 2):469-84. doi: 10.1113/jphysiol.2001.013453. J Physiol. 2002. PMID: 11956337 Free PMC article.
-
Phasic characteristic of elementary Ca(2+) release sites underlies quantal responses to IP(3).EMBO J. 2000 Jul 17;19(14):3608-17. doi: 10.1093/emboj/19.14.3608. EMBO J. 2000. PMID: 10899115 Free PMC article.
-
Modulation of IP(3)-sensitive Ca(2+) release by 2,3-butanedione monoxime.Pflugers Arch. 2003 Feb;445(5):614-21. doi: 10.1007/s00424-002-0984-9. Epub 2002 Dec 6. Pflugers Arch. 2003. PMID: 12634934
-
A bimodal pattern of InsP(3)-evoked elementary Ca(2+) signals in pancreatic acinar cells.Biophys J. 2000 May;78(5):2298-306. doi: 10.1016/S0006-3495(00)76776-2. Biophys J. 2000. PMID: 10777728 Free PMC article.
References
-
- Berridge MJ, Dupont G. Spatial and temporal signalling by calcium. Current Opinion in Cell Biology. 1994;6:267–274. - PubMed
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
-
- Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

