Low [ATP] and elevated [Mg2+] reduce depolarization-induced Ca2+ release in rat skinned skeletal muscle fibres
- PMID: 10517812
- PMCID: PMC2269557
- DOI: 10.1111/j.1469-7793.1999.00203.x
Low [ATP] and elevated [Mg2+] reduce depolarization-induced Ca2+ release in rat skinned skeletal muscle fibres
Abstract
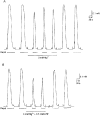
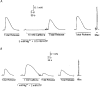
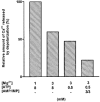
1. This study examined whether reduced [ATP], raised [Mg2+] and the presence of the metabolites AMP and inosine monophosphate (IMP) affected depolarization-induced Ca2+ release from the sarcoplasmic reticulum (SR) in mechanically skinned skeletal muscle fibres of the rat. The amount of Ca2+ released was determined from the extent of SR Ca2+ depletion following a depolarization in the specified conditions with 2 mM free EGTA present to chelate released Ca2+. 2. In the presence of 8 mM total ATP and 1 mM free Mg2+, most of the SR Ca2+ could be released by a single (2-3 s) depolarization. Paired comparisons in the same fibres showed that raising the [Mg2+] from 1 to 3 mM reduced the total amount of Ca2+ released by a single depolarization by approximately 40 %. At 1 mM Mg2+, lowering the [ATP] to 0.5 mM did not cause a detectable change in the total amount of Ca2+ released, but when the release rate was reduced by the presence of 3 mM Mg2+, lowering the [ATP] to 0.5 mM resulted in a further ( approximately 20 %) reduction in the total amount of Ca2+ released. 3. At 1 mM Mg2+ and 0.5 mM ATP, neither the presence of 3 mM AMP alone nor 3 mM AMP plus 3 mM IMP caused a significant change in total Ca2+ release. Furthermore, at 1 mM Mg2+, the combined effect of lowering the [ATP] from 8 to 0.5 mM and simultaneously adding 3 mM AMP and 3 mM IMP did not significantly alter total Ca2+ release. However, when Ca2+ release was already reduced by the presence of 3 mM Mg2+ and 0.5 mM ATP (to approximately 50 %), addition of 3 mM AMP and 3 mM IMP significantly reduced the amount of Ca2+ released a further 2-fold. 4. These results show that depolarization-induced Ca2+ release in mammalian muscle fibres is modulated by the concentration of ATP and its metabolic products, as well as by the free [Mg2+]. Consequently, the (reversible) reduction in Ca2+ release occurring in a muscle fibre after prolonged exercise could result not only from raised [Mg2+] but also from a severe reduction in [ATP] locally near the Ca2+ release channels, with the accompanying build-up of AMP and IMP further exacerbating this effect.
Figures
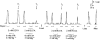
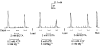
References
-
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. - PubMed
-
- Allen DG, Lännergren J, Westerblad H. Caged compounds affect calcium release in intact mouse skeletal muscle fibres. Proceedings of the Australian Physiological and Pharmacological Society. 1997b;28:15P.
-
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

