Fast skeletal muscle troponin T increases the cooperativity of transgenic mouse cardiac muscle contraction
- PMID: 10517814
- PMCID: PMC2269565
- DOI: 10.1111/j.1469-7793.1999.00231.x
Fast skeletal muscle troponin T increases the cooperativity of transgenic mouse cardiac muscle contraction
Abstract
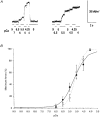
1. To investigate the functional significance of different troponin T (TnT) isoforms in the Ca2+ activation of muscle contraction, transgenic mice have been constructed with a chicken fast skeletal muscle TnT transgene driven by a cardiac alpha-myosin heavy chain gene promoter. 2. Cardiac muscle-specific expression of the fast skeletal muscle TnT has been obtained with significant myofibril incorporation. Expression of the endogenous cardiac muscle thin filament regulatory proteins, such as troponin I and tropomyosin, was not altered in the transgenic mouse heart, providing an authentic system for the functional characterization of TnT isoforms. 3. Cardiac muscle contractility was analysed for the force vs. Ca2+ relationship in skinned ventricular trabeculae of transgenic mice in comparison with wild-type litter-mates. The results showed unchanged pCa50 values (5.1 +/- 0.04 and 5.1 +/- 0.1, respectively) but significantly steeper slopes (the Hill coefficient was 2.0 +/- 0.2 vs. 1.0 +/- 0.2, P < 0.05). 4. The results demonstrate that the structural and functional variation of different TnT isoforms may contribute to the difference in responsiveness and overall cooperativity of the thin filament-based Ca2+ regulation between cardiac and skeletal muscles.
Figures


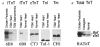
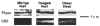

Similar articles
-
Troponin T isoforms alter the tolerance of transgenic mouse cardiac muscle to acidosis.Arch Biochem Biophys. 2004 Oct 15;430(2):178-84. doi: 10.1016/j.abb.2004.07.014. Arch Biochem Biophys. 2004. PMID: 15369816
-
Acidic and basic troponin T isoforms in mature fast-twitch skeletal muscle and effect on contractility.Am J Physiol. 1999 May;276(5):C1162-70. doi: 10.1152/ajpcell.1999.276.5.C1162. Am J Physiol. 1999. PMID: 10329966
-
Roles of troponin isoforms in pH dependence of contraction in rabbit fast and slow skeletal and cardiac muscles.J Biochem. 1999 Jul;126(1):121-9. doi: 10.1093/oxfordjournals.jbchem.a022412. J Biochem. 1999. PMID: 10393329
-
Evolution, Regulation, and Function of N-terminal Variable Region of Troponin T: Modulation of Muscle Contractility and Beyond.Int Rev Cell Mol Biol. 2016;321:1-28. doi: 10.1016/bs.ircmb.2015.09.002. Epub 2015 Nov 4. Int Rev Cell Mol Biol. 2016. PMID: 26811285 Review.
-
Troponin T: genetics, properties and function.J Muscle Res Cell Motil. 1998 Aug;19(6):575-602. doi: 10.1023/a:1005397501968. J Muscle Res Cell Motil. 1998. PMID: 9742444 Review.
Cited by
-
Nonmyofilament-associated troponin T fragments induce apoptosis.Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H283-92. doi: 10.1152/ajpheart.01200.2008. Epub 2009 Apr 24. Am J Physiol Heart Circ Physiol. 2009. PMID: 19395545 Free PMC article.
-
Troponin T core structure and the regulatory NH2-terminal variable region.Biochemistry. 2007 Feb 6;46(5):1368-79. doi: 10.1021/bi061949m. Biochemistry. 2007. PMID: 17260966 Free PMC article.
-
Abnormal splicing in the N-terminal variable region of cardiac troponin T impairs systolic function of the heart with preserved Frank-Starling compensation.Physiol Rep. 2014 Sep 4;2(9):e12139. doi: 10.14814/phy2.12139. Print 2014 Sep 1. Physiol Rep. 2014. PMID: 25194024 Free PMC article.
-
Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function.Arch Biochem Biophys. 2011 Jan 15;505(2):144-54. doi: 10.1016/j.abb.2010.10.013. Epub 2010 Oct 18. Arch Biochem Biophys. 2011. PMID: 20965144 Free PMC article. Review.
-
Genomic and functional analysis of the host response to acute simian varicella infection in the lung.Sci Rep. 2016 Sep 28;6:34164. doi: 10.1038/srep34164. Sci Rep. 2016. PMID: 27677639 Free PMC article.
References
-
- Akella AB, Ding X-L, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T band shifts in the diabetic rat. Circulation Research. 1995;76:600–606. - PubMed
-
- Anderson PAW, Greig A, Mark TA, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circulation Research. 1995;76:681–686. - PubMed
-
- Babu A, Scordilis SP, Sonnenblick EH, Gulati J. The control of myocardial contraction with skeletal fast muscle troponin C. Journal of Biological Chemistry. 1987;262:5815–5822. - PubMed
-
- Brandt PW, Diamond MS, Schachat FH. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. Journal of Molecular Biology. 1984;180:379–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

