Dynamic properties of endogenous phytochrome A in Arabidopsis seedlings
- PMID: 10517849
- PMCID: PMC59420
- DOI: 10.1104/pp.121.2.571
Dynamic properties of endogenous phytochrome A in Arabidopsis seedlings
Abstract
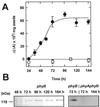
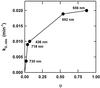
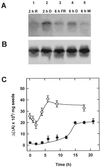
The dynamic behavior of phytochrome A (phyA) in seedlings of the model plant Arabidopsis was examined by in vivo spectroscopy and by western and northern blotting. Rapid accumulation of phyA was observed, reaching a steady state after 3 d. Both red and far-red light initiated a rapid destruction of the far-red-light-absorbing form of phytochrome (Pfr); the apparent half-life was only 4-fold longer in far-red than in red light. Furthermore, the Pfr-induced destruction of the red-light-absorbing form of phytochrome (Pr) of phyA occurred in darkness with a rate identical to that of Pfr destruction. A 2-fold decrease in mRNA abundance was observed after irradiation, irrespective of the applied light quality. However, reaccumulation occurred rapidly after far-red but slowly after red irradiation, indicating different modes of regulation of phytochrome expression after light-dark transitions depending on the light quality of the preceding irradiation. The wavelength dependency of the destruction rates was distinct from that of mustard, a close relative of Arabidopsis, and was explained on the basis of Pfr-induced Pr destruction and a simple kinetic two-step model. No dark reversion was detectable in the destruction kinetics after a red pulse. From these data we conclude that Arabidopsis phyA differs significantly in several aspects from other dicot phytochromes.
Figures




Similar articles
-
Phytochrome A enhances the promotion of hypocotyl growth caused by reductions in levels of phytochrome B in its far-red-light-absorbing form in light-grown Arabidopsis thaliana.Plant Physiol. 1996 Nov;112(3):965-73. doi: 10.1104/pp.112.3.965. Plant Physiol. 1996. PMID: 8938405 Free PMC article.
-
Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response.Plant Cell. 2009 Feb;21(2):494-506. doi: 10.1105/tpc.108.061259. Epub 2009 Feb 10. Plant Cell. 2009. PMID: 19208901 Free PMC article.
-
Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis.Plant Physiol. 1999 Dec;121(4):1207-16. doi: 10.1104/pp.121.4.1207. Plant Physiol. 1999. PMID: 10594107 Free PMC article.
-
The system of phytochromes: photobiophysics and photobiochemistry in vivo.Membr Cell Biol. 1998;12(5):691-720. Membr Cell Biol. 1998. PMID: 10379648 Review.
-
Thermal Reversion of Plant Phytochromes.Mol Plant. 2020 Mar 2;13(3):386-397. doi: 10.1016/j.molp.2019.12.004. Epub 2019 Dec 6. Mol Plant. 2020. PMID: 31812690 Review.
Cited by
-
eid1: a new Arabidopsis mutant hypersensitive in phytochrome A-dependent high-irradiance responses.Plant Cell. 2000 Apr;12(4):547-58. Plant Cell. 2000. PMID: 10760243 Free PMC article.
-
The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels.Plant Cell. 2008 Feb;20(2):337-52. doi: 10.1105/tpc.107.052142. Epub 2008 Feb 5. Plant Cell. 2008. PMID: 18252845 Free PMC article.
-
Resolving Structural Changes of Photoreceptors in Living Escherichia coli via In-cell Infrared Difference Spectroscopy.Bio Protoc. 2021 Feb 5;11(3):e3909. doi: 10.21769/BioProtoc.3909. eCollection 2021 Feb 5. Bio Protoc. 2021. PMID: 33732796 Free PMC article.
-
RSF1, an Arabidopsis locus implicated in phytochrome A signaling.Plant Physiol. 2000 Sep;124(1):39-45. doi: 10.1104/pp.124.1.39. Plant Physiol. 2000. PMID: 10982420 Free PMC article.
-
PCH1 and PCHL promote photomorphogenesis in plants by controlling phytochrome B dark reversion.Nat Commun. 2017 Dec 20;8(1):2221. doi: 10.1038/s41467-017-02311-8. Nat Commun. 2017. PMID: 29263319 Free PMC article.
References
-
- Briggs W, Rice HV. Phytochrome: chemical and physical properties and mechanism of action. Annu Rev Plant Physiol. 1972;23:293–334.
-
- Brockmann J, Rieble S, Kazarinova-Fukshansky N, Seyfried M, Schäfer E. Phytochrome behaves as a dimer in vivo. Plant Cell Environ. 1987;10:105–111.
-
- Casal JJ, Sánchez RA, Botto JF. Modes of action of phytochromes. J Exp Bot. 1998;49:127–138.
-
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. - PubMed
-
- Clough RC, Vierstra RD. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

