Rates of sugar uptake by guard cell protoplasts of pisum sativum L. Related To the solute requirement for stomatal opening
- PMID: 10517857
- PMCID: PMC59428
- DOI: 10.1104/pp.121.2.647
Rates of sugar uptake by guard cell protoplasts of pisum sativum L. Related To the solute requirement for stomatal opening
Abstract
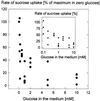
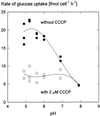
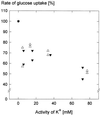
We wished to determine whether the capacity of the sugar uptake mechanisms of guard cells of the Argenteum mutant of pea (Pisum sativum L.) sufficed to support a concurrent stomatal opening movement. Sugar uptake by guard cell protoplasts was determined by silicone-oil-filtering centrifugation. The protoplasts took up [(14)C]glucose, [(14)C]fructose, and [(14)C]sucrose (Suc), apparently in symport with protons. Mannose, galactose, and fructose competed with Glc for transport by a presumed hexose carrier. The uptake of Glc saturated with a K(m) of 0.12 mM and a V(max) of 19 fmol cell(-1) h(-1). At external concentrations <1 mM, the uptake of Suc was slower than that of Glc. It exhibited a saturating component with a K(m) varying between 0.25 and 0.8 mM and a V(max) between 1 and 10 fmol cell(-1) h(-1), and at external concentrations >1 mM, a non-saturating component. At apoplastic sugar concentrations below 4 mM, sugar import was estimated to be mainly in the form of hexoses and too slow to support a simultaneous stomatal opening movement. If, however, during times of high photosynthesis and transpiration, the apoplastic Suc concentration rose and entered the range of non-saturating import, absorbed Suc could replace potassium malate as the osmoticum for the maintenance of stomatal opening.
Figures




Similar articles
-
Phosphate Translocator of Isolated Guard-Cell Chloroplasts from Pisum sativum L. Transports Glucose-6-Phosphate.Plant Physiol. 1993 Apr;101(4):1201-1207. doi: 10.1104/pp.101.4.1201. Plant Physiol. 1993. PMID: 12231774 Free PMC article.
-
Rubisco activity in guard cells compared with the solute requirement for stomatal opening.Plant Physiol. 1990 Jan;92(1):246-53. doi: 10.1104/pp.92.1.246. Plant Physiol. 1990. PMID: 16667255 Free PMC article.
-
Sugar uptake and proton release by protoplasts from the infected zone of Vicia faba L. nodules: evidence against apoplastic sugar supply of infected cells.J Exp Bot. 2003 Jul;54(388):1691-700. doi: 10.1093/jxb/erg191. Epub 2003 May 28. J Exp Bot. 2003. PMID: 12773525
-
Studying guard cells in the intact plant: modulation of stomatal movement by apoplastic factors.New Phytol. 2002 Mar;153(3):425-431. doi: 10.1046/j.0028-646X.2001.Documedoc.doc.x. New Phytol. 2002. PMID: 33863230 Review.
-
Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration?J Exp Bot. 2004 Sep;55(405):1963-76. doi: 10.1093/jxb/erh212. Epub 2004 Aug 13. J Exp Bot. 2004. PMID: 15310824 Review.
Cited by
-
Metabolite export of isolated guard cell chloroplasts of Vicia faba.New Phytol. 2003 Jul;159(1):195-202. doi: 10.1046/j.1469-8137.2003.00789.x. New Phytol. 2003. PMID: 33873676
-
Multi-level modeling of light-induced stomatal opening offers new insights into its regulation by drought.PLoS Comput Biol. 2014 Nov 13;10(11):e1003930. doi: 10.1371/journal.pcbi.1003930. eCollection 2014 Nov. PLoS Comput Biol. 2014. PMID: 25393147 Free PMC article.
-
Grape ASR Regulates Glucose Transport, Metabolism and Signaling.Int J Mol Sci. 2022 May 31;23(11):6194. doi: 10.3390/ijms23116194. Int J Mol Sci. 2022. PMID: 35682874 Free PMC article.
-
Relationships of Leaf Net Photosynthesis, Stomatal Conductance, and Mesophyll Conductance to Primary Metabolism: A Multispecies Meta-Analysis Approach.Plant Physiol. 2016 May;171(1):265-79. doi: 10.1104/pp.15.01660. Epub 2016 Mar 14. Plant Physiol. 2016. PMID: 26977088 Free PMC article. Review.
-
Channelling auxin action: modulation of ion transport by indole-3-acetic acid.Plant Mol Biol. 2002 Jun-Jul;49(3-4):349-56. Plant Mol Biol. 2002. PMID: 12036259 Review.
References
-
- Aked J, Hall JL. The uptake of glucose, fructose and sucrose into the lower epidermis of leaf discs of pea (Pisum sativum L. cv. Argenteum) New Phytol. 1993;123:271–276.
-
- Boorer KJ, Loo DDF, Frommer WB, Wright EM. Transport mechanism of the cloned potato H+-sucrose cotransporter StSUT1. J Biol Chem. 1996;271:25139–25144. - PubMed
-
- Borchert S, Grosse H, Heldt HW. Specific transport of inorganic phosphate, dihydroxyacetone phosphate and 3-phosphoglycerate into amyloplasts from pea roots. FEBS Lett. 1989;253:183–186.
-
- Buckhout TJ. Sucrose transport in isolated plasma-membrane vesicles from sugar beet (Beta vulgaris L.): evidence for an electrogenic sucrose-proton symport. Planta. 1989;178:393–399. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

