BRCA1-associated growth arrest is RB-dependent
- PMID: 10518542
- PMCID: PMC18378
- DOI: 10.1073/pnas.96.21.11866
BRCA1-associated growth arrest is RB-dependent
Abstract
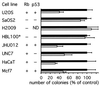
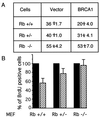
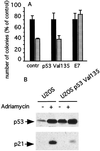
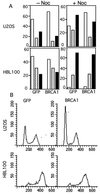
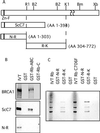
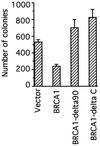
BRCA1 is a susceptibility gene for breast and ovarian cancer with growth-inhibitory activity for which the mechanism of action remains unclear. When introduced into cells, BRCA1 inhibits growth of some but not all cell lines. In an attempt to uncover the mechanism of growth suppression by BRCA1, we examined a panel of cell lines for their ability to reduce colony outgrowth in response to BRCA1 overexpression. Of all variables tested, only those cells with wild-type pRb were sensitive to BRCA1-induced growth suppression. In cells with an intact rb gene, inactivation of pRb by HPV E7 abrogates the growth arrest imposed by BRCA1. In accordance with these observations, we found that BRCA1 could not suppress BrdUrd uptake in primary fibroblasts from rb-/- mice and exhibited an intermediate ability to inhibit DNA synthesis in rb+/- as compared with rb+/+ cells. We further found that the BRCA1 protein complexes with the hypophosphorylated form of pRb. This binding is localized to amino acids 304-394 of BRCA1 protein and requires the ABC domain of pRb. In-frame deletion of BRCA1 fragment involved in interaction with pRb completely abolished the growth-suppressive property of BRCA1. Although it has been reported that BRCA1 interacts with p53, we find the p53 status did not affect the ability of BRCA1 to suppress colony formation. Our data suggest that the growth suppressor function of BRCA1 depends, at least in part, on Rb.
Figures


References
-
- Thompson M E, Jensen R A, Obermiller P S, Page D L, Holt J T. Nat Genet. 1995;9:444–450. - PubMed
-
- Holt J T, Thompson M E, Szabo C, Robinson-Benion C, Arteaga C, King M-C, Jensen R. Nat Genet. 1996;12:298–302. - PubMed
-
- Gudas J M, Nguyen H, Li T, Cowan K H. Cancer Res. 1995;55:4561–4565. - PubMed
-
- Vaughn J P, Davis P L, Japboe M D, Huper G, Evans A C, Wiseman R W, Berchuck A, Iglehart J D, Futreal P A, Marks J R. Cell Growth Differ. 1996;7:711–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

