Fgfr2 is required for limb outgrowth and lung-branching morphogenesis
- PMID: 10518547
- PMCID: PMC18383
- DOI: 10.1073/pnas.96.21.11895
Fgfr2 is required for limb outgrowth and lung-branching morphogenesis
Abstract
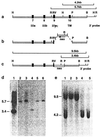
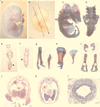
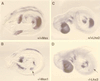
The aim of this study was to clarify the role of Fgfr2 during later stages of embryonic development. Of two previously reported gene-targeting experiments, the more extensive Fgfr2 deletion was lethal shortly after implantation, because of trophoblast defects, whereas the less extensive one survived until midgestation with placental insufficiency and defective limb outgrowth [Xu, X., Weinstein, M., Li, C., Naski, M., Cohen, R. I., Ornitz, D. M., Leder, P. & Deng, C. (1998) Development (Cambridge, U.K.) 125, 753-765]. Fgfr2 in the early embryo is expressed in the trophectoderm, and this extra-embryonic localization persists into mid- and late gestation, when Fgfr2 also is expressed in multiple developing organs. To gain insight into the later functions of Fgfr2, fusion chimeras were constructed from homozygous mutant embryonic stem cells and wild-type tetraploid embryos. This allowed survival until term and revealed that Fgfr2 is required for both limb outgrowth and branching lung morphogenesis. The use of fusion chimeras demonstrated that early lethality was indeed because of trophectoderm defects and indicated that in the embryonic cell lineages Fgfr2 activity manifests in limb and lung development. Highly similar lung and limb phenotypes were detected recently in the loss of function mutation of Fgf10, a ligand of Fgfr2. It is likely, therefore, that whereas during early development Fgfr2 interacts with Fgf4, in limb and lung development interactions between Fgf10 and Fgfr2 may be required. Possible epithelial-mesenchymal interactions between the splicing alternatives of Fgfr2 and their specific ligands will be discussed.
Figures

References
-
- Cohn M J, Izpisua-Belmonte J C, Abud H, Heath J K, Tickle C. Cell. 1995;80:739–746. - PubMed
-
- Crossley P H, Minowada G, MacArthur C A, Martin G R. Cell. 1996;84:127–136. - PubMed
-
- Ohuchi H, Nakagawa T, Yamamoto A, Araga A, Ohata T, Ishimaru Y, Yoshioka H, Kuwana T, Nohno T, Yamasaki M, et al. Development (Cambridge, UK) 1997;124:2235–2244. - PubMed
-
- Abud H, Skinner J A, McDonald F J, Bedford M T, Lonai P, Heath J K. Dev Genet. 1996;181:51–65. - PubMed
-
- Martin G R. Genes Dev. 1998;12:1571–1586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

