Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism
- PMID: 10518597
- PMCID: PMC18433
- DOI: 10.1073/pnas.96.21.12186
Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism
Abstract
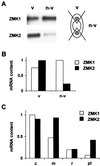
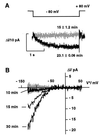
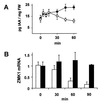
Auxin-induced growth of coleoptiles depends on the presence of potassium and is suppressed by K+ channel blockers. To evaluate the role of K+ channels in auxin-mediated growth, we isolated and functionally expressed ZMK1 and ZMK2 (Zea mays K+ channel 1 and 2), two potassium channels from maize coleoptiles. In growth experiments, the time course of auxin-induced expression of ZMK1 coincided with the kinetics of coleoptile elongation. Upon gravistimulation of maize seedlings, ZMK1 expression followed the gravitropic-induced auxin redistribution. K+ channel expression increased even before a bending of the coleoptile was observed. The transcript level of ZMK2, expressed in vascular tissue, was not affected by auxin. In patch-clamp studies on coleoptile protoplasts, auxin increased K+ channel density while leaving channel properties unaffected. Thus, we conclude that coleoptile growth depends on the transcriptional up-regulation of ZMK1, an inwardly rectifying K+ channel expressed in the nonvascular tissue of this organ.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

