The gamma-crystallins and human cataracts: a puzzle made clearer
- PMID: 10521291
- PMCID: PMC1288278
- DOI: 10.1086/302619
The gamma-crystallins and human cataracts: a puzzle made clearer
Abstract
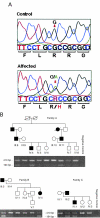
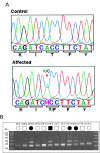
Despite the fact that cataracts constitute the leading cause of blindness worldwide, the mechanisms of lens opacification remain unclear. We recently mapped the aculeiform cataract to the gamma-crystallin locus (CRYG) on chromosome 2q33-35, and mutational analysis of the CRYG-genes cluster identified the aculeiform-cataract mutation in exon 2 of gamma-crystallin D (CRYGD). This mutation occurred in a highly conserved amino acid and could be associated with an impaired folding of CRYGD. During our study, we observed that the previously reported Coppock-like-cataract mutation, the first human cataract mutation, in the pseudogene CRYGE represented a polymorphism seen in 23% of our control population. Further analysis of the original Coppock-like-cataract family identified a missense mutation in a highly conserved segment of exon 2 of CRYGC. These mutations were not seen in a large control population. There is no direct evidence, to date, that up-regulation of a pseudogene causes cataracts. To our knowledge, these findings are the first evidence of an involvement of CRYGC and support the role of CRYGD in human cataract formation.
Figures
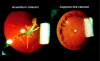
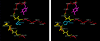
References
Electronic-Database Information
-
- Searching GenBank, http://www.ncbi.nlm.nih.gov/Genbank/GenbankSearch.html (for sequence information on crygb and CRYGC [M19364 and K03003, respectively], CRYGD [K03005 and K03006], and CRYGE [S72943 and K03008])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for aculeiform cataract [MIM 115700], Coppock-like cataract [MIM 123660 and MIM 116200], and progressive polymorphic cataract [MIM 601286])
-
- SWISS-MODEL, http://www.expasy.ch/swissmod/SWISS-MODEL.html (for protein-homology modeling)
References
-
- Brakenhoff R, Henskens H, van Rossum M, Lubsen N, Schoenmakers G (1994) Activation of the γE-crystallin pseudogene in the human hereditary Coppock-like cataract. Hum Mol Genet 3:279–283 - PubMed
-
- Cartier M, Breitman M, Tsui L-C (1992) A frame-shift mutation in the gammaE-crystallin gene of the Elo mouse. Nat Genet 2:42–45 - PubMed
-
- Cartier M, Tsui L, Ball S, Lubsen N (1994) Crystallins genes and cataract. In: Wright A, Jay B (eds) Modern genetics. Vol 2: Molecular genetics of inherited eye disorders. Harwood Academic, Edinburgh, pp 413–443
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

