Functional expression of tagged human Na+-glucose cotransporter in Xenopus laevis oocytes
- PMID: 10523405
- PMCID: PMC2269588
- DOI: 10.1111/j.1469-7793.1999.00359.x
Functional expression of tagged human Na+-glucose cotransporter in Xenopus laevis oocytes
Abstract
1. High-affinity, secondary active transport of glucose in the intestine and kidney is mediated by an integral membrane protein named SGLT1 (sodium glucose cotransporter). Though basic properties of the transporter are now defined, many questions regarding the structure- function relationship of the protein, its biosynthesis and targeting remain unanswered. In order to better address these questions, we produced a functional hSGLT1 protein (from human) containing a reporter tag. 2. Six constructs, made from three tags (myc, haemaglutinin and poly-His) inserted at both the C- and N-terminal positions, were thus tested using the Xenopus oocyte expression system via electrophysiology and immunohistochemistry. Of these, only the hSGLT1 construct with the myc tag inserted at the N-terminal position proved to be of interest, all other constructs showing no or little transport activity. A systematic comparison of transport properties was therefore performed between the myc-tagged and the untagged hSGLT1 proteins. 3. On the basis of both steady-state (affinities for substrate (glucose) and inhibitor (phlorizin) as well as expression levels) and presteady-state parameters (transient currents) we conclude that the two proteins are functionally indistinguishable, at least under these criteria. Immunological detection confirmed the appropriate targeting of the tagged protein to the plasma membrane of the oocyte with the epitope located at the extracellular side. 4. The myc-tagged hSGLT1 was also successfully expressed in polarized MDCK cells. alpha-Methylglucose uptake studies on transfected cells showed an exclusively apical uptake pathway, thus indicating that the expressed protein was correctly targeted to the apical domain of the cell. 5. These comparative studies demonstrate that the myc epitope inserted at the N-terminus of hSGLT1 produces a fully functional protein while other epitopes of similar size inserted at either end of the protein inactivated the final protein.
Figures



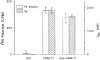
 , corresponding values of currents elicited by addition of 5 m
, corresponding values of currents elicited by addition of 5 m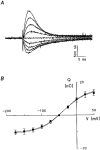

 ) were tested for currents induced by various sugars (Man, mannitol; 2-DG, 2-deoxyglucose; 3-OMG, 3-O-methylglucose; α-MG, α-methylglucose; and GLC, glucose. Oocytes injected with 5 ng cRNA were tested on day 5 with 5 m
) were tested for currents induced by various sugars (Man, mannitol; 2-DG, 2-deoxyglucose; 3-OMG, 3-O-methylglucose; α-MG, α-methylglucose; and GLC, glucose. Oocytes injected with 5 ng cRNA were tested on day 5 with 5 m

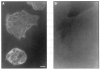

 ) uptake. The non-specific uptake, as determined by addition of 0.2 m
) uptake. The non-specific uptake, as determined by addition of 0.2 mReferences
-
- Bissonnette P, Gagné H, Blais A, Berteloot A. 2-Deoxyglucose transport and metabolism in Caco-2 cells. American Journal of Physiology. 1996a;270:G153–162. - PubMed
-
- Bissonnette P, Gagné H, Coady MJ, Benabdallah K, Lapointe JY, Berteloot A. Kinetic separation and characterisation of three sugar transport modes in Caco-2 cells. American Journal of Physiology. 1996b;270:G833–843. - PubMed
-
- Blais A, Bissonnette P, Berteloot A. Common characteristics for Na+-dependent sugar transport in Caco-2 cells and human fetal colon. Journal of Membrane Biology. 1987;99:113–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

