Nitric oxide increases persistent sodium current in rat hippocampal neurons
- PMID: 10523414
- PMCID: PMC2269585
- DOI: 10.1111/j.1469-7793.1999.t01-1-00451.x
Nitric oxide increases persistent sodium current in rat hippocampal neurons
Abstract
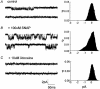
1. The effects of nitric oxide (NO) donors on whole-cell, TTX-sensitive sodium currents and single sodium channels in excised patches were examined in rat hippocampal neurons. The whole-cell sodium current consisted of a large transient component (INa,t) and a smaller, inactivation-resistant, persistent component (INa,p). 2. In acutely dissociated neurons, the amplitude of the whole-cell INa, p increased by 60-80 % within a few minutes of exposure to either of two NO donors, sodium nitroprusside (SNP, 100 microM) or S-nitroso-N-acetyl-DL-penicillamine (SNAP, 100 microM). 3. The amplitude of INa,t was not changed significantly by the same concentrations of SNP and SNAP, indicating that NO had a selective effect on INa,p. 4. Both NO donors significantly increased the mean persistent current in excised inside-out patches from cultured hippocampal neurons. SNP at 10-100 microM increased average mean persistent current at a pipette potential (Vp) of +30 mV from -0.010 +/- 0.014 pA (control) to -2.91 +/- 1.41 pA (n = 10). SNAP at 3-100 microM increased the average mean inward current in six inside-out patches from -0.07 +/- 0.02 to -0.30 +/- 0.08 pA (Vp = +30 mV). 5. The increase in persistent Na+ channel activity recorded in inside-out patches in the presence of SNP or SNAP could be reversed by the reducing agent dithiothreitol (DTT, 2-5 mM) or by lidocaine (1-10 microM). 6. The average mean current recorded in the presence of SNP was 10-fold higher than that elicited by SNAP. The time delay before an increase was observed was shorter with SNP (4.0 +/- 0.8 min, n = 8) than with SNAP (8.4 +/- 1.6 min, n = 7). 7. A component of the SNP molecule added on its own, 5 mM sodium cyanide (NaCN), increased mean current in excised inside-out patches (Vp = +30 mV) from -0.06 +/- 0.04 to -0.58 +/- 0.21 pA (n = 19). This increase in channel activity could be blocked by 10 microM lidocaine and 2-5 mM DTT. 8. These results suggest that NO may directly increase the activity of neuronal persistent Na+ channels, but not transient Na+ channels, through an oxidizing action directly on the channel protein or on a closely associated regulatory protein in the plasma membrane.
Figures

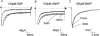
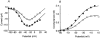



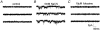


References
-
- Alonso A, Llinás RR. Subthreshold Na+-dependent theta-like rythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:1175–1177. - PubMed
-
- Amitai Y. Membrane potential oscillations underlying firing patterns in neocortical neurons. Neuroscience. 1994;63:151–161. - PubMed
-
- Arden SR, Sinor JD, Potthoff WK, Aizenman E. Subunit-specific interactions of cyanide with the N-methyl-D-aspartate receptor. Journal of Biological Chemistry. 1998;273:21505–21511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

