Effects of calcium buffering on glucose-induced insulin release in mouse pancreatic islets: an approximation to the calcium sensor
- PMID: 10523416
- PMCID: PMC2269605
- DOI: 10.1111/j.1469-7793.1999.00473.x
Effects of calcium buffering on glucose-induced insulin release in mouse pancreatic islets: an approximation to the calcium sensor
Abstract
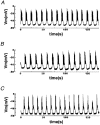
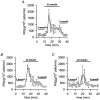
1. The properties of the calcium sensor for glucose-induced insulin secretion have been studied using cell-permeant Ca2+ buffers with distinct kinetics and affinities. In addition, submembrane cytosolic Ca2+ distribution has been modelled after trains of glucose-induced action potential-like depolarizations. 2. Slow Ca2+ buffers (around 1 mmol l-1 intracellular concentration) with different affinities (EGTA and Calcium Orange-5N) did not significantly affect glucose-induced insulin release. Modelling showed no effect on cytosolic Ca2+ concentrations at the outermost shell (0.05 microm), their effects being observed in the innermost shells dependent on Ca2+ affinity. 3. In contrast, fast Ca2+ buffers (around 1 mmol l-1 intracellular concentration) with different affinities (BAPTA and Calcium Green-5N) caused a 50 % inhibition of early insulin response and completely blocked the late phase of glucose-induced insulin response, their simulations showing a decrease of [Ca2+]i at both the inner and outermost shells. 4. These data are consistent with the existence in pancreatic beta-cells of a higher affinity Ca2+ sensor than that proposed for neurons. Moreover, these data are consistent with the proposed existence of two distinct pools of granules: (i) 'primed' vesicles, colocalized with Ca2+ channels and responsible of the first phase of insulin release; and (ii) 'reserved pool' vesicles, not colocalized and responsible for the second phase.
Figures




References
-
- Ashcroft FM, Harrison DE, Ashcroft SJH. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature. 1984;312:446–448. - PubMed
-
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Progress in Biophysics and Molecular Biology. 1989;54:87–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

