Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper
- PMID: 10523566
- PMCID: PMC85712
- DOI: 10.1128/JCM.37.11.3634-3643.1999
Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper
Abstract
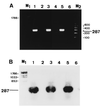
Reverse transcription-PCR (RT-PCR) was used to detect canine distemper virus (CDV) nucleoprotein (NP) RNA in serum, whole blood, and cerebrospinal fluid (CSF) samples from 38 dogs with clinically suspected distemper. Results were correlated to clinical findings, anti-CDV neutralizing antibody titers, postmortem findings, and demonstration of CDV NP antigen by immunohistochemistry. The specificity of the RT-PCR was ensured by amplification of RNA from various laboratory CDV strains, restriction enzyme digestion, and Southern blot hybridization. In 29 of 38 dogs, CDV infection was confirmed by postmortem examination and immunohistochemistry. The animals displayed the catarrhal, systemic, and nervous forms of distemper. Seventeen samples (serum, whole blood, or CSF) from dogs with distemper were tested with three sets of primers targeted to different regions of the NP gene of the CDV Onderstepoort strain. Expected amplicons were observed in 82, 53, and 41% of the 17 samples, depending upon the primer pair used. With the most sensitive primer pair (primer pair I), CDV NP RNA was detected in 25 of 29 (86%) serum samples and 14 of 16 (88%) whole blood and CSF samples from dogs with distemper but not in body fluids from immunohistochemically negative dogs. Nucleotide sequence analysis of five RT-PCR amplicons from isolates from the field revealed few silent point mutations. These isolates exhibited greater homology to the Rockborn (97 to 99%) than to the Onderstepoort (95 to 96%) CDV strain. In summary, although the sensitivity of the RT-PCR for detection of CDV is strongly influenced by the location of the selected primers, this nucleic acid detection system represents a highly specific and sensitive method for the antemortem diagnosis of distemper in dogs, regardless of the form of distemper, humoral immune response, and viral antigen distribution.
Figures





References
-
- Alldinger S, Baumgärtner W, van Moll P, Örvell C. In vivo and in vitro expression of canine distemper viral proteins in dogs and non-domestic carnivores. Arch Virol. 1993;132:421–428. - PubMed
-
- Alldinger S, Wünschmann A, Baumgärtner W, Voss C, Kremmer E. Up-regulation of major histocompatibility complex class II antigen expression in the central nervous system of dogs with spontaneous canine distemper virus encephalitis. Acta Neuropathol. 1996;92:273–280. - PubMed
-
- Appel M. Canine distemper virus. In: Appel M J G, editor. Virus infections of carnivores. Amsterdam, The Netherlands: Elsevier Science Publishers; 1987. pp. 133–159.
-
- Appel M J G, Gillespie J H. Canine distemper virus. In: Gard S, Hallauer C, Meyer K F, editors. Virology monographs 11. New York, N.Y: Springer-Verlag; 1972. pp. 1–96.
-
- Axthelm M K, Krakowka S. Canine distemper virus: the early blood-brain barrier lesion. Acta Neuropathol. 1987;75:27–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

