Effects of OspA vaccination on Lyme disease serologic testing
- PMID: 10523583
- PMCID: PMC85737
- DOI: 10.1128/JCM.37.11.3718-3721.1999
Effects of OspA vaccination on Lyme disease serologic testing
Abstract
This study presents the effects of OspA vaccination on two-step testing for Borrelia burgdorferi antibodies. Although vaccinees developed enzyme-linked immunosorbent assay reactivity, immunoblots did not fulfill Centers for Disease Control and Prevention criteria for positivity. Furthermore, OspA reactivity did not interfere with interpretation of immunoblots with sera from patients who developed early Lyme disease despite vaccination.
Figures

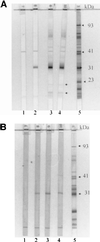



References
-
- American College of Physicians. Guidelines for the laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1106–1108. - PubMed
-
- Anonymous. Lyme disease vaccine approved. JAMA. 1999;281:405. - PubMed
-
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. - PubMed
-
- Johnson B J B, Robbins K E, Bailey R E, Cao B-L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

