Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros
- PMID: 10523602
- PMCID: PMC2195663
- DOI: 10.1084/jem.190.8.1039
Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros
Abstract
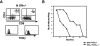
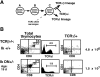
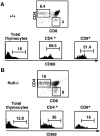
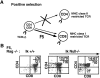
T cell differentiation relies on pre-T cell receptor (TCR) and TCR signaling events that take place at successive steps of the pathway. Here, we show that two of these T cell differentiation checkpoints are regulated by Ikaros. In the absence of Ikaros, double negative thymocytes can differentiate to the double positive stage without expression of a pre-TCR complex. Subsequent events in T cell development mediated by TCR involving transition from the double positive to the single positive stage are also regulated by Ikaros. Nonetheless, in Ikaros-deficient thymocytes, the requirement of pre-TCR expression for expansion of immature thymocytes as they progress to the double positive stage is still maintained, and the T cell malignancies that invariably arise in the thymus of Ikaros-deficient mice are dependent on either pre-TCR or TCR signaling. We conclude that Ikaros regulates T cell differentiation, selection, and homeostasis by providing signaling thresholds for pre-TCR and TCR.
Figures





References
-
- Godfrey D.I., Kennedy J., Mombaerts P., Tonegawa S., Zlotnik A. Onset of TCR-β gene rearrangement and role of TCR-β expression during CD3−CD4−CD8− thymocyte differentiation. J. Immunol. 1994;152:4783–4792. - PubMed
-
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. - PubMed
-
- Mombaerts P., Iacomini J., Johnson R., Herrup K., Tonegawa S., Papaioannou V. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. - PubMed
-
- Mombaerts P., Clarke A., Rudnicki M., Iacomini J., Itohara S., Lafaille J., Wang L., Ichikawa Y., Jaenisch R., Hooper M., Tonegawa S. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages [published erratum at 360:491] Nature. 1992;360:225–231. - PubMed
-
- Nossal G. Negative selection of lymphocytes. Cell. 1994;76:229–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

