In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines
- PMID: 10523610
- PMCID: PMC2195660
- DOI: 10.1084/jem.190.8.1123
In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines
Abstract
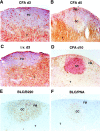
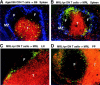
Migration of antigen-activated CD4 T cells to B cell areas of lymphoid tissues is important for mounting T cell-dependent antibody responses. Here we show that CXC chemokine receptor (CXCR)5, the receptor for B lymphocyte chemoattractant (BLC), is upregulated on antigen-specific CD4 T cells in vivo when animals are immunized under conditions that promote T cell migration to follicles. In situ hybridization of secondary follicles for BLC showed high expression in mantle zones and low expression in germinal centers. When tested directly ex vivo, CXCR5(hi) T cells exhibited a vigorous chemotactic response to BLC. At the same time, the CXCR5(hi) cells showed reduced responsiveness to the T zone chemokines, Epstein-Barr virus-induced molecule 1 (EBI-1) ligand chemokine (ELC) and secondary lymphoid tissue chemokine (SLC). After adoptive transfer, CXCR5(hi) CD4 T cells did not migrate to follicles, indicating that additional changes may occur after immunization that help direct T cells to follicles. To further explore whether T cells could acquire an intrinsic ability to migrate to follicles, CD4(-)CD8(-) double negative (DN) T cells from MRL-lpr mice were studied. These T cells normally accumulate within follicles of MRL-lpr mice. Upon transfer to wild-type recipients, DN T cells migrated to follicle proximal regions in all secondary lymphoid tissues. Taken together, our findings indicate that reprogramming of responsiveness to constitutively expressed lymphoid tissue chemokines plays an important role in T cell migration to the B cell compartment of lymphoid tissues.
Figures



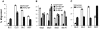
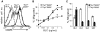
References
-
- MacLennan I.C.M. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. - PubMed
-
- Kelsoe G. The germinal centera crucible for lymphocyte selection. Semin. Immunol. 1996;8:179–184. - PubMed
-
- Cyster J.G. Signaling thresholds and interclonal competition in preimmune B-cell selection. Immunol. Rev. 1997;156:87–101. - PubMed
-
- Van den Eertwegh A.J.M., Noelle R.J., Roy M., Shepherd D.M., Aruffo A., Ledbetter J.A., Boersma W.J.A., Claassen E. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J. Exp. Med. 1993;178:1555–1565. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

