An open reading frame element mediates posttranscriptional regulation of tropoelastin and responsiveness to transforming growth factor beta1
- PMID: 10523620
- PMCID: PMC84725
- DOI: 10.1128/MCB.19.11.7314
An open reading frame element mediates posttranscriptional regulation of tropoelastin and responsiveness to transforming growth factor beta1
Abstract
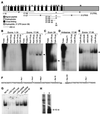
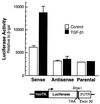
Elastin, an extracellular component of arteries, lung, and skin, is produced during fetal and neonatal growth. We reported previously that the cessation of elastin production is controlled by a posttranscriptional mechanism. Although tropoelastin pre-mRNA is transcribed at the same rate in neonates and adults, marked instability of the fully processed transcript bars protein production in mature tissue. Using RNase protection, we identified a 10-nucleotide sequence in tropoelastin mRNA near the 5' end of the sequences coded by exon 30 that interacts specifically with a developmentally regulated cytosolic 50-kDa protein. Binding activity increased as tropoelastin expression dropped, being low in neonatal fibroblasts and high in adult cells, and treatment with transforming growth factor beta1 (TGF-beta1), which stimulates tropoelastin expression by stabilizing its mRNA, reduced mRNA-binding activity. No other region of tropoelastin mRNA interacted with cellular proteins, and no binding activity was detected in nuclear extracts. The ability of the exon-30 element to control mRNA decay and responsiveness to TGF-beta1 was assessed by three distinct functional assays: (i) insertion of exon 30 into a heterologous gene conferred increased reporter activity after exposure to TGF-beta1; (ii) addition of excess exon 30 RNA slowed tropoelastin mRNA decay in an in vitro polysome degradation assay; and (iii) a mutant tropoelastin cDNA lacking exon 30, compared to wild-type cDNA, produced a stable transcript whose levels were not affected by TGF-beta1. These findings demonstrate that posttranscriptional regulation of elastin production in mature tissue is conferred by a specific element within the open reading frame of tropoelastin mRNA.
Figures






References
-
- Bandziulis R J, Swanson M S, Dreyfus G. RNA-binding proteins as developmental regulators. Genes Dev. 1989;3:431–437. - PubMed
-
- Baumann C, Xirasagar S, Gollnick P. The trp RNA-binding attenuation protein (TRAP) from Bacillus subtilis binds to unstacked trp leader RNA. J Biol Chem. 1997;272:19863–19869. - PubMed
-
- Baurén G, Wieslander L. Splicing of Balbiani ring 1 gene pre-mRNA occurs simultaneously with transcription. Cell. 1994;76:183–192. - PubMed
-
- Belasco J, Brawerman G, editors. Control of messenger RNA stability. San Diego, Calif: Academic Press, Inc.; 1993.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
