Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import
- PMID: 10523667
- PMCID: PMC84838
- DOI: 10.1128/MCB.19.11.7782
Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import
Abstract
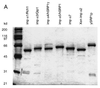
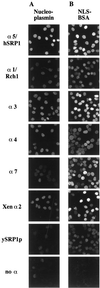
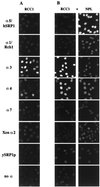
Importin alpha plays a pivotal role in the classical nuclear protein import pathway. Importin alpha shuttles between nucleus and cytoplasm, binds nuclear localization signal-bearing proteins, and functions as an adapter to access the importin beta-dependent import pathway. In contrast to what is found for importin beta, several isoforms of importin alpha, which can be grouped into three subfamilies, exist in higher eucaryotes. We describe here a novel member of the human family, importin alpha7. To analyze specific functions of the distinct importin alpha proteins, we recombinantly expressed and purified five human importin alpha's along with importin alpha from Xenopus and Saccharomyces cerevisiae. Binding affinity studies showed that all importin alpha proteins from humans or Xenopus bind their import receptor (importin beta) and their export receptor (CAS) with only marginal differences. Using an in vitro import assay based on permeabilized HeLa cells, we compared the import substrate specificities of the various importin alpha proteins. When the substrates were tested singly, only the import of RCC1 showed a strong preference for one family member, importin alpha3, whereas most of the other substrates were imported by all importin alpha proteins with similar efficiencies. However, strikingly different substrate preferences of the various importin alpha proteins were revealed when two substrates were offered simultaneously.
Figures






References
-
- Bischoff F R, Postingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991;354:80–82. - PubMed
-
- Conti E, Uy M, Leighton L, Blobel G, Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin α. Cell. 1998;94:193–204. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
