CREB-independent regulation by CBP is a novel mechanism of human growth hormone gene expression
- PMID: 10525051
- PMCID: PMC408577
- DOI: 10.1172/JCI7308
CREB-independent regulation by CBP is a novel mechanism of human growth hormone gene expression
Abstract
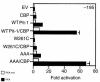
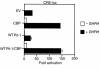
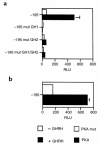
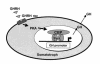
Hypothalamic growth hormone-releasing hormone (GHRH) stimulates growth hormone (GH) gene expression in anterior pituitary somatotrophs by binding to the GHRH receptor, a G-protein-coupled transmembrane receptor, and by mediating a cAMP-mediated protein kinase A (PKA) signal-transduction pathway. Two nonclassical cAMP-response element motifs (CGTCA) are located at nucleotides -187/-183 (distal cAMP-response element; dCRE) and -99/-95 (proximal cAMP-response element; pCRE) of the human GH promoter and are required for cAMP responsiveness, along with the pituitary-specific transcription factor Pit-1 (official nomenclature, POU1F1). Although a role for cAMP-response element binding protein (CREB) in GH stimulation by PKA has been suggested, it is unclear how the effect may be mediated. CREB binding protein (CBP) is a nuclear cofactor named for its ability to bind CREB. However, CBP also binds other nuclear proteins. We determined that CBP interacts with Pit-1 and is a cofactor for Pit-1-dependent activation of the human GH promoter. This pathway appears to be independent of CREB, with CPB being the likely target of phosphorylation by PKA.
Figures



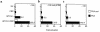
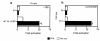
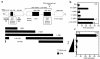

Similar articles
-
Two CGTCA motifs and a GHF1/Pit1 binding site mediate cAMP-dependent protein kinase A regulation of human growth hormone gene expression in rat anterior pituitary GC cells.J Biol Chem. 1994 Jan 21;269(3):1804-14. J Biol Chem. 1994. PMID: 8294429
-
A novel mechanism for cyclic adenosine 3',5'-monophosphate regulation of gene expression by CREB-binding protein.Mol Endocrinol. 1999 Feb;13(2):268-75. doi: 10.1210/mend.13.2.0245. Mol Endocrinol. 1999. PMID: 9973256
-
cAMP response element-binding protein-binding protein mediates thyrotropin-releasing hormone signaling on thyrotropin subunit genes.J Biol Chem. 2000 Oct 27;275(43):33365-72. doi: 10.1074/jbc.M006819200. J Biol Chem. 2000. PMID: 10931853
-
Nuclear effects of the cAMP pathway activation in somatotrophs.Horm Res. 1997;47(4-6):245-50. doi: 10.1159/000185471. Horm Res. 1997. PMID: 9167959 Review.
-
Regulation of somatostatin gene transcription by cyclic adenosine monophosphate.Metabolism. 1996 Aug;45(8 Suppl 1):4-7. doi: 10.1016/s0026-0495(96)90068-2. Metabolism. 1996. PMID: 8769368 Review.
Cited by
-
Loss of CREB Coactivator CRTC1 in SF1 Cells Leads to Hyperphagia and Obesity by High-fat Diet But Not Normal Chow Diet.Endocrinology. 2021 Sep 1;162(9):bqab076. doi: 10.1210/endocr/bqab076. Endocrinology. 2021. PMID: 33846709 Free PMC article.
-
Structural basis for activation of the growth hormone-releasing hormone receptor.Nat Commun. 2020 Oct 15;11(1):5205. doi: 10.1038/s41467-020-18945-0. Nat Commun. 2020. PMID: 33060564 Free PMC article.
-
Short-chain fatty acids inhibit growth hormone and prolactin gene transcription via cAMP/PKA/CREB signaling pathway in dairy cow anterior pituitary cells.Int J Mol Sci. 2013 Oct 30;14(11):21474-88. doi: 10.3390/ijms141121474. Int J Mol Sci. 2013. PMID: 24177567 Free PMC article.
-
Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18303-8. doi: 10.1073/pnas.1421815112. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489106 Free PMC article.
-
Update on regulation of GHRH and its actions on GH secretion in health and disease.Rev Endocr Metab Disord. 2025 Jun;26(3):305-320. doi: 10.1007/s11154-025-09943-y. Epub 2025 Jan 21. Rev Endocr Metab Disord. 2025. PMID: 39838154 Review.
References
-
- Nelson C, Albert VR, Elsholtz HP, Lu LI, Rosenfeld MG. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988;239:1400–1405. - PubMed
-
- Bodner M, et al. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988;55:505–518. - PubMed
-
- Fox SR, et al. The homeodomain protein, Pit-1/GHF-1, is capable of binding to and activating cell-specific elements of both the growth hormone and prolactin gene promoters. Mol Endocrinol. 1990;4:1069–1080. - PubMed
-
- Ingraham HA, et al. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55:519–529. - PubMed
-
- Theill LE, Castrillo JL, Wu D, Karin M. Dissection of functional domains of the pituitary-specific transcription factor GHF-1. Nature. 1989;342:945–948. - PubMed

