Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride
- PMID: 10525052
- PMCID: PMC408575
- DOI: 10.1172/JCI6786
Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride
Abstract
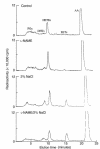
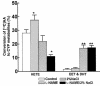
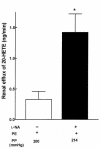
Renal function is perturbed by inhibition of nitric oxide synthase (NOS). To probe the basis of this effect, we characterized the effects of nitric oxide (NO), a known suppressor of cytochrome P450 (CYP) enzymes, on metabolism of arachidonic acid (AA), the expression of omega-hydroxylase, and the efflux of 20-hydroxyeicosatetraenoic acid (20-HETE) from the isolated kidney. The capacity to convert [(14)C]AA to HETEs and epoxides (EETs) was greater in cortical microsomes than in medullary microsomes. Sodium nitroprusside (10-100 microM), an NO donor, inhibited renal microsomal conversion of [(14)C]AA to HETEs and EETs in a dose-dependent manner. 8-bromo cGMP (100 microM), the cell-permeable analogue of cGMP, did not affect conversion of [(14)C]AA. Inhibition of NOS with N(omega)-nitro-L-arginine-methyl ester (L-NAME) significantly increased conversion of [(14)C]AA to HETE and greatly increased the expression of omega-hydroxylase protein, but this treatment had only a modest effect on epoxygenase activity. L-NAME induced a 4-fold increase in renal efflux of 20-HETE, as did L-nitroarginine. Oral treatment with 2% sodium chloride (NaCl) for 7 days increased renal epoxygenase activity, both in the cortex and the medulla. In contrast, cortical omega-hydroxylase activity was reduced by treatment with 2% NaCl. Coadministration of L-NAME and 2% NaCl decreased conversion of [(14)C]AA to HETEs without affecting epoxygenase activity. Thus, inhibition of NOS increased omega-hydroxylase activity, CYP4A expression, and renal efflux of 20-HETE, whereas 2% NaCl stimulated epoxygenase activity.
Figures


References
-
- Moncada S, Palmer RMG, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. - PubMed
-
- Nathan C. Nitric oxide is a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. - PubMed
-
- Hurshman AR, Marletta MA. Nitric oxide complexes of inducible nitric oxide synthase: spectral characterization and effects on catalytic activity. Biochemistry. 1995;34:5627–5634. - PubMed
-
- Griscavage JM, Habbs AJ, Ignarro LJ. Negative modulation of nitric oxide synthase by nitric oxide and nitroso compounds. Adv Pharmacol. 1995;34:215–234. - PubMed

