Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes
- PMID: 10525053
- PMCID: PMC408571
- DOI: 10.1172/JCI6310
Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes
Abstract
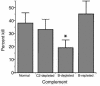
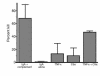
The role of IgA in the control of invasive mucosal pathogens such as Streptococcus pneumoniae is poorly understood. We demonstrate that human pneumococcal capsular polysaccharide-specific IgA initiated dose-dependent killing of S. pneumoniae with complement and phagocytes. The majority of specific IgA in serum was of the polymeric form (pIgA), and the efficiency of pIgA-initiated killing exceeded that of monomeric IgA-initiated killing. In the absence of complement, specific IgA induced minimal bacterial adherence, uptake, and killing. Killing of S. pneumoniae by resting phagocytes with immune IgA required complement, predominantly via the C2-independent alternative pathway, which requires factor B, but not calcium. Both S. pneumoniae-bound IgA and complement were involved, as demonstrated by a 50% decrease in killing with blocking of Fcalpha receptor (CD89) and CR1/CR3 (CD35/CD11b). However, IgA-mediated killing by phagocytes could be reproduced in the absence of opsonic complement by pre-activating phagocytes with the inflammatory products C5a and TNF-alpha. Thus, S. pneumoniae capsule-specific IgA may show distinct roles in effecting clearance of S. pneumoniae in the presence or absence of inflammation. These data suggest mechanisms whereby pIgA may serve to control pneumococcal infections locally and upon the pathogen's entry into the bloodstream.
Figures
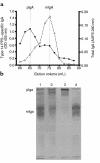
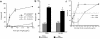
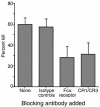
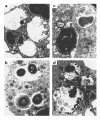

Similar articles
-
Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae.Infect Immun. 2007 Apr;75(4):1801-10. doi: 10.1128/IAI.01758-06. Epub 2007 Jan 29. Infect Immun. 2007. PMID: 17261616 Free PMC article.
-
Prolonged and preferential production of polymeric immunoglobulin A in response to Streptococcus pneumoniae capsular polysaccharides.Infect Immun. 1996 Oct;64(10):4339-44. doi: 10.1128/iai.64.10.4339-4344.1996. Infect Immun. 1996. PMID: 8926108 Free PMC article.
-
Induction of functional secretory IgA responses in breast milk, by pneumococcal capsular polysaccharides.J Infect Dis. 2002 Nov 15;186(10):1422-9. doi: 10.1086/344356. Epub 2002 Oct 23. J Infect Dis. 2002. PMID: 12404157
-
Immunoglobulin A: interaction with complement, phagocytic cells and endothelial cells.Complement Inflamm. 1991;8(5-6):347-58. doi: 10.1159/000463206. Complement Inflamm. 1991. PMID: 1802552 Review.
-
The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream.Rev Infect Dis. 1983 Sep-Oct;5 Suppl 4:S797-805. doi: 10.1093/clinids/5.supplement_4.s797. Rev Infect Dis. 1983. PMID: 6356294 Review.
Cited by
-
A molecular mucosal adjuvant to enhance immunity against pneumococcal infection in the elderly.Immune Netw. 2015 Feb;15(1):9-15. doi: 10.4110/in.2015.15.1.9. Epub 2015 Feb 17. Immune Netw. 2015. PMID: 25713504 Free PMC article. Review.
-
Modulation of the lung inflammatory response to serotype 8 pneumococcal infection by a human immunoglobulin m monoclonal antibody to serotype 8 capsular polysaccharide.Infect Immun. 2005 Aug;73(8):4530-8. doi: 10.1128/IAI.73.8.4530-4538.2005. Infect Immun. 2005. PMID: 16040964 Free PMC article.
-
Modulation of polymorphonuclear cell interleukin-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide.Infect Immun. 2003 Dec;71(12):6775-83. doi: 10.1128/IAI.71.12.6775-6783.2003. Infect Immun. 2003. PMID: 14638763 Free PMC article.
-
Bacterium-like Particles from Corynebacterium pseudodiphtheriticum as Mucosal Adjuvant for the Development of Pneumococcal Vaccines.Vaccines (Basel). 2024 Apr 12;12(4):412. doi: 10.3390/vaccines12040412. Vaccines (Basel). 2024. PMID: 38675794 Free PMC article.
-
Primary pneumococcal peritonitis can be the first presentation of a familial complement factor I deficiency1.Clin Exp Immunol. 2020 Dec;202(3):379-383. doi: 10.1111/cei.13490. Epub 2020 Jul 24. Clin Exp Immunol. 2020. PMID: 32640035 Free PMC article.
References
-
- Russell, M.W., Kilian, M., and Lamm, M.E. 1999. Biological activities of IgA. In Mucosal immunology. 2nd edition. P.L. Ogra et al., editors. Academic Press. San Diego, CA. 225–240.
-
- Underdown BJ, Schiff JM. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. - PubMed
-
- Mestecky, J., and McGhee, J.R. 1987. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. In Advances in immunology. Volume 40. F.J. Dixon, editor. Academic Press. San Diego, CA. 153–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

