RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes
- PMID: 10525529
- PMCID: PMC2174216
- DOI: 10.1083/jcb.147.2.207
RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes
Abstract
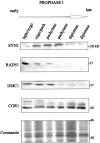
The eukaryotic RecA homologues RAD51 and DMC1 function in homology recognition and formation of joint-molecule recombination intermediates during yeast meiosis. The precise immunolocalization of these two proteins on the meiotic chromosomes of plants and animals has been complicated by their high degree of identity at the amino acid level. With antibodies that have been immunodepleted of cross-reactive epitopes, we demonstrate that RAD51 and DMC1 have identical distribution patterns in extracts of mouse spermatocytes in successive prophase I stages, suggesting coordinate functionality. Immunofluorescence and immunoelectron microscopy with these antibodies demonstrate colocalization of the two proteins on the meiotic chromosome cores at early prophase I. We also show that mouse RAD51 and DMC1 establish protein-protein interactions with each other and with the chromosome core component COR1(SCP3) in a two-hybrid system and in vitro binding analyses. These results suggest that the formation of a multiprotein recombination complex associated with the meiotic chromosome cores is essential for the development and fulfillment of the meiotic recombination process.
Figures






References
-
- Albini S.M., Jones G.H. Synaptonemal complex spreading in Allium cepa and A. fistulosum. The initiation and sequence of pairing. Chromosoma. 1987;95:324–338.
-
- Anderson L.K., Stack S.M. Nodules associated with axial cores and synaptonemal complexes during zygotene in Psilotum nudum . Chromosoma. 1988;97:96–100.
-
- Baker S.M., Plug A.W., Prolla T.A., Bronner C.E., Harris A.C., Yao X., Christie D.M., Monell C., Arnheim N., Bradley A., Ashley T., Liskay R.M. Involvement of mouse MLH1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 1996;13:336–342. - PubMed
-
- Barlow C., Hirotsune S., Paylor R., Liyanage M., Eckhaus M., Collins F., Shiloh Y., Crawley J.N., Ried T., Tagle D., Wynshaw-Boris A. Atm-deficient micea paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

