Glycosylation can influence topogenesis of membrane proteins and reveals dynamic reorientation of nascent polypeptides within the translocon
- PMID: 10525533
- PMCID: PMC2174215
- DOI: 10.1083/jcb.147.2.257
Glycosylation can influence topogenesis of membrane proteins and reveals dynamic reorientation of nascent polypeptides within the translocon
Abstract
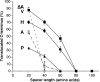
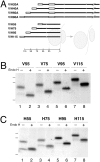
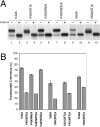
The topology of multispanning membrane proteins in the mammalian endoplasmic reticulum is thought to be dictated primarily by the first hydrophobic sequence. We analyzed the in vivo insertion of a series of chimeric model proteins containing two conflicting signal sequences, i.e., an NH(2)-terminal and an internal signal, each of which normally directs translocation of its COOH-terminal end. When the signals were separated by more than 60 residues, linear insertion with the second signal acting as a stop-transfer sequence was observed. With shorter spacers, an increasing fraction of proteins inserted with a translocated COOH terminus as dictated by the second signal. Whether this resulted from membrane targeting via the second signal was tested by measuring the targeting efficiency of NH(2)-terminal signals followed by polypeptides of different lengths. The results show that targeting is mediated predominantly by the first signal in a protein. Most importantly, we discovered that glycosylation within the spacer sequence affects protein orientation. This indicates that the nascent polypeptide can reorient within the translocation machinery, a process that is blocked by glycosylation. Thus, topogenesis of membrane proteins is a dynamic process in which topogenic information of closely spaced signal and transmembrane sequences is integrated.
Figures




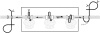
References
-
- Andrews D.W., Young J.C., Mirels L.F., Czarnota G.J. The role of the N-region in signal sequence and signal-anchor function. J. Biol. Chem. 1992;267:7761–7769. - PubMed
-
- Beltzer J.P., Fiedler K., Fuhrer C., Geffen I., Handschin C., Wessels H.P., Spiess M. Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J. Biol. Chem. 1991;266:973–978. - PubMed
-
- Coleman J., Inukai M., Inouye M. Dual functions of the signal peptide in protein transfer across the membrane. Cell. 1985;43:351–360. - PubMed

