Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor
- PMID: 10525547
- PMCID: PMC2174214
- DOI: 10.1083/jcb.147.2.447
Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor
Abstract
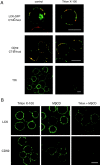
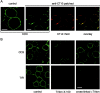
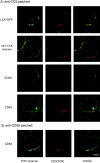
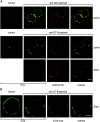
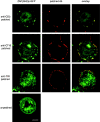
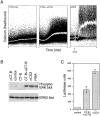
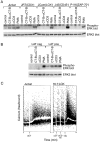
The role of lipid rafts in T cell antigen receptor (TCR) signaling was investigated using fluorescence microscopy. Lipid rafts labeled with cholera toxin B subunit (CT-B) and cross-linked into patches displayed characteristics of rafts isolated biochemically, including detergent resistance and colocalization with raft-associated proteins. LCK, LAT, and the TCR all colocalized with lipid patches, although TCR association was sensitive to nonionic detergent. Aggregation of the TCR by anti-CD3 mAb cross-linking also caused coaggregation of raft-associated proteins. However, the protein tyrosine phosphatase CD45 did not colocalize to either CT-B or CD3 patches. Cross-linking of either CD3 or CT-B strongly induced tyrosine phosphorylation and recruitment of a ZAP-70(SH2)(2)-green fluorescent protein (GFP) fusion protein to the lipid patches. Also, CT-B patching induced signaling events analagous to TCR stimulation, with the same dependence on expression of key TCR signaling molecules. Targeting of LCK to rafts was necessary for these events, as a nonraft- associated transmembrane LCK chimera, which did not colocalize with TCR patches, could not reconstitute CT-B-induced signaling. Thus, our results indicate a mechanism whereby TCR engagement promotes aggregation of lipid rafts, which facilitates colocalization of LCK, LAT, and the TCR whilst excluding CD45, thereby triggering protein tyrosine phosphorylation.
Figures



References
-
- Anderson R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
-
- Boniface J.J., Rabinowitz J.D., Wulfing C., Hampl J., Reich Z., Altman J.D., Kantor R.M., Beeson C., McConnell H.M., Davis M.M. Initiation of signal transduction through the T cell receptor requires the peptide multivalent engagement of MHC ligands. Immunity. 1998;9:459–466. - PubMed
-
- Brdicka T., Cerny J., Horejsi V. T cell receptor signalling results in rapid tyrosine phosphorylation of the linker protein LAT present in detergent-resistant membrane microdomains. Biochem. Biophys. Res. Commun. 1998;248:356–360. - PubMed
-
- Brown D. The tyrosine kinase connectionhow GPI-anchored proteins activate T cells. Curr. Opin. Immunol. 1993;5:349–354. - PubMed
-
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

