Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice
- PMID: 10531279
- PMCID: PMC97005
- DOI: 10.1128/IAI.67.11.6152-6156.1999
Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice
Abstract
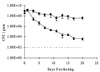
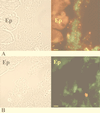
The role of the Klebsiella pneumoniae capsular polysaccharide (K antigen) during colonization of the mouse large intestine was assessed with wild-type K. pneumoniae LM21 and its isogenic capsule-defective mutant. When bacterial strains were fed alone to mice, the capsulated bacteria persisted in the intestinal tract at levels of 10(8) CFU/g of feces while the capsule-defective strain colonized at low levels, 10(4) CFU/g of feces. In mixed-infection experiments, the mutant was rapidly outcompeted by the wild type. In situ hybridization on colonic sections revealed that bacterial cells of both strains were evenly distributed in the mucus layer at day 1 after infection, while at day 20 the wild type remained dispersed and the capsule-defective strain was seen in clusters in the mucus layer. These results suggest that capsular polysaccharide plays an important role in the gut colonization ability of K. pneumoniae.
Figures
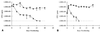

Similar articles
-
Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells.Infect Immun. 1999 Feb;67(2):554-61. doi: 10.1128/IAI.67.2.554-561.1999. Infect Immun. 1999. PMID: 9916058 Free PMC article.
-
In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent smooth strain of Salmonella typhimurium and its lipopolysaccharide-deficient mutant.Infect Immun. 1987 Dec;55(12):2884-90. doi: 10.1128/iai.55.12.2884-2890.1987. Infect Immun. 1987. PMID: 3316026 Free PMC article.
-
The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Klebsiella pneumoniae.Microb Pathog. 1993 Jun;14(6):433-40. doi: 10.1006/mpat.1993.1042. Microb Pathog. 1993. PMID: 7692209
-
Klebsiella pneumoniae: Going on the Offense with a Strong Defense.Microbiol Mol Biol Rev. 2016 Jun 15;80(3):629-61. doi: 10.1128/MMBR.00078-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27307579 Free PMC article. Review.
-
Structure, assembly and regulation of expression of capsules in Escherichia coli.Mol Microbiol. 1999 Mar;31(5):1307-19. doi: 10.1046/j.1365-2958.1999.01276.x. Mol Microbiol. 1999. PMID: 10200953 Review.
Cited by
-
Animal Model To Study Klebsiella pneumoniae Gastrointestinal Colonization and Host-to-Host Transmission.Infect Immun. 2020 Oct 19;88(11):e00071-20. doi: 10.1128/IAI.00071-20. Print 2020 Oct 19. Infect Immun. 2020. PMID: 32839189 Free PMC article.
-
Surveillance and Genomic Analysis of Third-Generation Cephalosporin-Resistant and Carbapenem-Resistant Klebsiella pneumoniae Complex in Germany.Antibiotics (Basel). 2022 Sep 21;11(10):1286. doi: 10.3390/antibiotics11101286. Antibiotics (Basel). 2022. PMID: 36289942 Free PMC article.
-
The Capsule Regulatory Network of Klebsiella pneumoniae Defined by density-TraDISort.mBio. 2018 Nov 20;9(6):e01863-18. doi: 10.1128/mBio.01863-18. mBio. 2018. PMID: 30459193 Free PMC article.
-
The Saposin-Like Protein AplD Displays Pore-Forming Activity and Participates in Defense Against Bacterial Infection During a Multicellular Stage of Dictyostelium discoideum.Front Cell Infect Microbiol. 2018 Mar 15;8:73. doi: 10.3389/fcimb.2018.00073. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29662839 Free PMC article.
-
Characterization of a DHA-1-producing Klebsiella pneumoniae strain involved in an outbreak and role of the AmpR regulator in virulence.Antimicrob Agents Chemother. 2012 Jan;56(1):288-94. doi: 10.1128/AAC.00164-11. Epub 2011 Oct 10. Antimicrob Agents Chemother. 2012. PMID: 21986829 Free PMC article.
References
-
- Camprubi S, Merino S, Guillot J F, Tomas J M. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Klebsiella pneumoniae. Microb Pathog. 1993;14:433–440. - PubMed
-
- Dechamps C, Sauvant M P, Chanal C, Sirot D, Gazui N, Malhuret R, Baguet J C, Sirot J. Prospective survey of colonization and infection caused by expanded-spectrum β-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J Clin Microbiol. 1989;27:2887–2890. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

