Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling
- PMID: 10531353
- PMCID: PMC3204605
- DOI: 10.1074/jbc.274.44.31506
Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling
Abstract
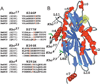
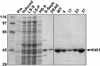
To study the relationship between conventional kinesin's structure and function, we identified 13 lethal mutations in the Drosophila kinesin heavy chain motor domain and tested a subset for effects on mechanochemistry. S246F is a moderate mutation that occurs in loop 11 between the ATP- and microtubule-binding sites. While ATP and microtubule binding appear normal, there is a 3-fold decrease in the rate of ATP turnover. This is consistent with the hypothesis that loop 11 provides a structural link that is important for the activation of ATP turnover by microtubule binding. T291M is a severe mutation that occurs in alpha-helix 5 near the center of the microtubule-binding surface. It impairs the microtubule-kinesin interaction and directly effects the ATP-binding pocket, allowing an increase in ATP turnover in the absence of microtubules. The T291M mutation may mimic the structure of a microtubule-bound, partially activated state. E164K is a moderate mutation that occurs at the beta-sheet 5a/loop 8b junction, remote from the ATP pocket. Surprisingly, it causes both tighter ATP-binding and a 2-fold decrease in ATP turnover. We propose that E164 forms an ionic bridge with alpha-helix 5 and speculate that it helps coordinate the alternating site catalysis of dimerized kinesin heavy chain motor domains.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

