Multiple and opposing roles of cholinergic transmission in the main olfactory bulb
- PMID: 10531421
- PMCID: PMC6782910
- DOI: 10.1523/JNEUROSCI.19-21-09180.1999
Multiple and opposing roles of cholinergic transmission in the main olfactory bulb
Abstract
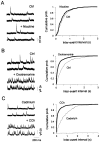
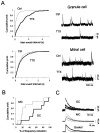
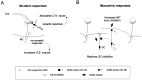
The main olfactory bulb is a critical relay step between the olfactory epithelium and the olfactory cortex. A marked feature of the bulb is its massive innervation by cholinergic inputs from the basal forebrain. In this study, we addressed the functional interaction between cholinergic inputs and intrinsic bulbar circuitry. Determining the roles of acetylcholine (ACh) requires the characterization of cholinergic effects on both neural excitability and synaptic transmission. For this purpose, we used electrophysiological techniques to localize and characterize the diverse roles of ACh in mouse olfactory bulb slices. We found that cholinergic inputs have a surprising number of target receptor populations that are expressed on three different neuronal types in the bulb. Specifically, nicotinic acetylcholine receptors excite both the output neurons of the bulb, i.e., the mitral cells, as well as interneurons located in the periglomerular regions. These nicotine-induced responses in interneurons are short lasting, whereas responses in mitral cells are long lasting. In contrast, muscarinic receptors have an inhibitory effect on the firing rate of interneurons from a deeper layer, granule cells, while at the same time they increase the degree of activity-independent transmitter release from these cells onto mitral cells. Cholinergic signaling thus was found to have multiple and opposing roles in the olfactory bulb. These dual cholinergic effects on mitral cells and interneurons may be important in modulating olfactory bulb output to central structures required for driven behaviors and may be relevant to understanding mechanisms underlying the perturbations of cholinergic inputs to cortex that occur in Alzheimer's disease.
Figures



Similar articles
-
Signaling between periglomerular cells reveals a bimodal role for GABA in modulating glomerular microcircuitry in the olfactory bulb.Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):9478-83. doi: 10.1073/pnas.1424406112. Epub 2015 Jul 13. Proc Natl Acad Sci U S A. 2015. PMID: 26170298 Free PMC article.
-
Cholinergic modulation of excitability in the rat olfactory bulb: effect of local application of cholinergic agents on evoked field potentials.Neuroscience. 1991;45(3):653-62. doi: 10.1016/0306-4522(91)90278-v. Neuroscience. 1991. PMID: 1775240
-
GABAA and glutamate receptor involvement in dendrodendritic synaptic interactions from salamander olfactory bulb.J Physiol. 1993 Sep;469:315-39. doi: 10.1113/jphysiol.1993.sp019816. J Physiol. 1993. PMID: 7903696 Free PMC article.
-
Developmental and aging aspects of the cholinergic innervation of the olfactory bulb.Int J Dev Neurosci. 1998 Nov-Dec;16(7-8):777-85. doi: 10.1016/s0736-5748(98)00087-2. Int J Dev Neurosci. 1998. PMID: 10198824 Review.
-
Neuronal organization of olfactory bulb circuits.Front Neural Circuits. 2014 Sep 3;8:98. doi: 10.3389/fncir.2014.00098. eCollection 2014. Front Neural Circuits. 2014. PMID: 25232305 Free PMC article. Review.
Cited by
-
Nicotinic receptors modulate olfactory bulb external tufted cells via an excitation-dependent inhibitory mechanism.J Neurophysiol. 2013 Oct;110(7):1544-53. doi: 10.1152/jn.00865.2012. Epub 2013 Jul 10. J Neurophysiol. 2013. PMID: 23843430 Free PMC article.
-
Neurogenesis drives stimulus decorrelation in a model of the olfactory bulb.PLoS Comput Biol. 2012;8(3):e1002398. doi: 10.1371/journal.pcbi.1002398. Epub 2012 Mar 15. PLoS Comput Biol. 2012. PMID: 22442645 Free PMC article.
-
The effect of bilirubin on the excitability of mitral cells in the olfactory bulb of the rat.Sci Rep. 2016 Sep 9;6:32872. doi: 10.1038/srep32872. Sci Rep. 2016. PMID: 27611599 Free PMC article.
-
Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb.J Neurosci. 2006 Mar 22;26(12):3292-8. doi: 10.1523/JNEUROSCI.4768-05.2006. J Neurosci. 2006. PMID: 16554479 Free PMC article.
-
Biophysical constraints on lateral inhibition in the olfactory bulb.J Neurophysiol. 2016 Jun 1;115(6):2937-49. doi: 10.1152/jn.00671.2015. Epub 2016 Mar 23. J Neurophysiol. 2016. PMID: 27009162 Free PMC article.
References
-
- Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69:711–726. - PubMed
-
- Alkondon M, Albuquerque EX. Presence of α-bungarotoxin-sensitive nicotinic ACh receptors in rat olfactory bulb neurons. Neurosci Lett. 1994;176:152–156. - PubMed
-
- Alkondon M, Rocha ES, Maelicke A, Albuquerque EX. Diversity of nicotinic ACh receptors in rat brain. V. α-bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther. 1996;278:1460–1471. - PubMed
-
- Brown DA, Abogadie FC, Allen TG, Buckley NJ, Caulfield MP, Delmas P, Haley JE, Lamas JA, Selyanko AA. Muscarinic mechanisms in nerve cells. Life Sci. 1997;60:1137–1144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
