Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS
- PMID: 10531444
- PMCID: PMC6782941
- DOI: 10.1523/JNEUROSCI.19-21-09399.1999
Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS
Abstract
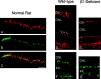
From the elegant studies of Ramon y Cajal (1909) to the current advances in molecular cloning (e.g., Farber and Danciger, 1997), the retina has served as an ideal model for the entire CNS. We have taken advantage of the well described anatomy, physiology, and molecular biology of the retina to begin to examine the role of the laminins, one component of the extracellular matrix, on the processes of neuronal differentiation and synapse formation in the CNS. We have examined the effect of the deletion of one laminin chain, the beta2 chain, on retinal development. The gross development of retinas from laminin beta2 chain-deficient animals appears normal, and photoreceptors are formed. However, these retinas exhibit several pathologies: laminin beta2 chain-deficient mice display abnormal outer segment elongation, abnormal electroretinograms, and abnormal rod photoreceptor synapses. Morphologically, the outer segments are reduced by 50% in length; the outer plexiform layer of mutant animals is disrupted specifically, because only 7% of observed rod invaginating synapses appear normal, whereas the inner plexiform layer is undisturbed; finally, the rate of apoptosis in the mutant photoreceptor layer is twice that of control mice. Physiologically, the electroretinogram is altered; the amplitude of the b-wave and the slope of the b-wave intensity-response function are both decreased, consistent with synaptic disruption in the outer retina. Together, these results emphasize the prominence of the extracellular matrix and, in particular, the laminins in the development and maintenance of synaptic function and morphogenesis in the CNS.
Figures







References
-
- Altshuler D, Cepko C. A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development. 1992;114:942–957. - PubMed
-
- Arahata K, Ishii H, Hayashi YK. Congenital muscular dystrophies. Curr Opin Neurol. 1995;8:385–390. - PubMed
-
- Balkema GW. A synaptic antigen (B16) is localized in retinal synaptic ribbons. J Comp Neurol. 1991;312:573–583. - PubMed
-
- Balkema GW, Rizkalla R. Ultrastructural localization of a synaptic ribbon protein recognized by antibody B16. J Neurocytol. 1996;25:565–571. - PubMed
-
- Blank M, Koulen P, Blake DJ, Kröger S. Dystrophin and beta-dystroglycan in photoreceptor terminals from normal and mdx3Cv mouse retinae. Eur J Neurosci. 1999;11:2121–2133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
