A protein residing at the subunit interface of the bacterial ribosome
- PMID: 10535924
- PMCID: PMC22919
- DOI: 10.1073/pnas.96.22.12345
A protein residing at the subunit interface of the bacterial ribosome
Abstract
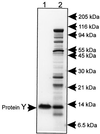
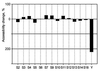
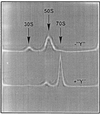
Surface labeling of Escherichia coli ribosomes with the use of the tritium bombardment technique has revealed a minor unidentified ribosome-bound protein (spot Y) that is hidden in the 70S ribosome and becomes highly labeled on dissociation of the 70S ribosome into subunits. In the present work, the N-terminal sequence of the protein Y was determined and its gene was identified as yfia, an ORF located upstream the phe operon of E. coli. This 12.7-kDa protein was isolated and characterized. An affinity of the purified protein Y for the 30S subunit, but not for the 50S ribosomal subunit, was shown. The protein proved to be exposed on the surface of the 30S subunit. The attachment of the 50S subunit resulted in hiding the protein Y, thus suggesting the protein location at the subunit interface in the 70S ribosome. The protein was shown to stabilize ribosomes against dissociation. The possible role of the protein Y as ribosome association factor in translation is discussed.
Figures



References
-
- Goldanskii V I, Kashirin I A, Shishkov A V, Baratova L A, Grebenshchikov N I. J Mol Biol. 1988;201:567–574. - PubMed
-
- Baratova L A, Grebenshchikov N I, Dobrov E N, Gedrovich A V, Kashirin I A, Shishkov A V, Efimov A V, Jarvekulg L, Radavsky Y L, Saarma M. Virology. 1992;188:175–180. - PubMed
-
- Baratova L A, Grebenshchikov N I, Shishkov A V, Kashirin I A, Radavsky J L, Jarvekulg L, Saarma M. J Gen Virol. 1992;73:229–235. - PubMed
-
- Tsetlin V I, Alyonycheva T N, Shemyakin V V, Neiman L A, Ivanov V T. Eur J Biochem. 1988;178:123–129. - PubMed
-
- Yusupov M M, Spirin A S. FEBS Lett. 1986;197:229–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

