Tropomyosin directly modulates actomyosin mechanical performance at the level of a single actin filament
- PMID: 10535949
- PMCID: PMC22958
- DOI: 10.1073/pnas.96.22.12488
Tropomyosin directly modulates actomyosin mechanical performance at the level of a single actin filament
Abstract
Muscle contraction is the result of myosin cross-bridges (XBs) cyclically interacting with the actin-containing thin filament. This interaction is modulated by the thin filament regulatory proteins, troponin and tropomyosin (Tm). With the use of an in vitro motility assay, the role of Tm in myosin's ability to generate force and motion was assessed. At saturating myosin surface densities, Tm had no effect on thin filament velocity. However, below 50% myosin saturation, a significant reduction in actin-Tm filament velocity was observed, with complete inhibition of movement occurring at 12. 5% of saturating surface densities. Under similar conditions, actin filaments alone demonstrated no reduction in velocity. The effect of Tm on force generation was assessed at the level of a single thin filament. In the absence of Tm, isometric force was a linear function of the density of myosin on the motility surface. At 50% myosin surface saturation, the presence of Tm resulted in a 2-fold enhancement of force relative to actin alone. However, no further potentiation of force was observed with Tm at saturating myosin surface densities. These results indicate that, in the presence of Tm, the strong binding of myosin cooperatively activates the thin filament. The inhibition of velocity at low myosin densities and the potentiation of force at higher myosin densities suggest that Tm can directly modulate the kinetics of a single myosin XB and the recruitment of a population of XBs, respectively. At saturating myosin conditions, Tm does not appear to affect the recruitment or the kinetics of myosin XBs.
Figures
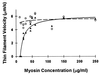

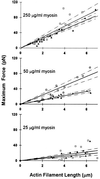
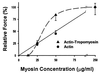
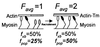
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

