A mechanism of paraquat toxicity involving nitric oxide synthase
- PMID: 10535996
- PMCID: PMC23088
- DOI: 10.1073/pnas.96.22.12760
A mechanism of paraquat toxicity involving nitric oxide synthase
Abstract
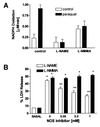
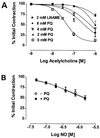
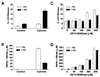
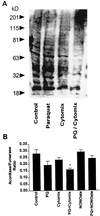
Paraquat (PQ) is a well described pneumotoxicant that produces toxicity by redox cycling with cellular diaphorases, thereby elevating intracellular levels of superoxide (O-(2)). NO synthase (NOS) has been shown to participate in PQ-induced lung injury. Current theory holds that NO reacts with O-(2) generated by PQ to produce the toxin peroxynitrite. We asked whether NOS might alternatively function as a PQ diaphorase and reexamined the question of whether NO/O-(2) reactions were toxic or protective. Here, we show that: (i) neuronal NOS has PQ diaphorase activity that inversely correlates with NO formation; (ii) PQ-induced endothelial cell toxicity is attenuated by inhibitors of NOS that prevent NADPH oxidation, but is not attenuated by those that do not; (iii) PQ inhibits endothelium-derived, but not NO-induced, relaxations of aortic rings; and (iv) PQ-induced cytotoxicity is potentiated in cytokine-activated macrophages in a manner that correlates with its ability to block NO formation. These data indicate that NOS is a PQ diaphorase and that toxicity of such redox-active compounds involves a loss of NO-related activity.
Figures

References
-
- Brooks R E. Lab Invest. 1971;25:536–545. - PubMed
-
- Bus J S, Aust S D, Gibson J E. Biochem Biophys Res Commun. 1974;58:749–755. - PubMed
-
- Dicker E, Cederbaum A I. Biochem Pharmacol. 1991;42:529–535. - PubMed
-
- Liochev S I, Fridovich I. Free Radical Biol Med. 1994;16:555–559. - PubMed
-
- Shimada H, Hirai K, Simamura E, Pan J. Arch Biochem Biophys. 1998;351:75–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

