Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor
- PMID: 10535997
- PMCID: PMC23090
- DOI: 10.1073/pnas.96.22.12766
Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor
Abstract
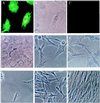
Endocytosis of the Flaviviridae viruses, hepatitis C virus, GB virus C/hepatitis G virus, and bovine viral diarrheal virus (BVDV) was shown to be mediated by low density lipoprotein (LDL) receptors on cultured cells by several lines of evidence: by the demonstration that endocytosis of these virus correlated with LDL receptor activity, by complete inhibition of detectable endocytosis by anti-LDL receptor antibody, by inhibition with anti-apolipoprotein E and -apolipoprotein B antibodies, by chemical methods abrogating lipoprotein/LDL receptor interactions, and by inhibition with the endocytosis inhibitor phenylarsine oxide. Confirmatory evidence was provided by the lack of detectable LDL receptor on cells known to be resistant to BVDV infection. Endocytosis via the LDL receptor was shown to be mediated by complexing of the virus to very low density lipoprotein or LDL but not high density lipoprotein. Studies using LDL receptor-deficient cells or a cytolytic BVDV system indicated that the LDL receptor may be the main but not exclusive means of cell entry of these viruses. Studies on other types of viruses indicated that this mechanism may not be exclusive to Flaviviridae but may be used by viruses that associate with lipoprotein in the blood. These findings provide evidence that the family of LDL receptors may serve as viral receptors.
Figures





References
-
- Agnello V, Chung R T, Kaplan L M. N Engl J Med. 1992;327:1490–5. - PubMed
-
- Agnello V. Springer Semin Immunopathol. 1997;19:111–129. - PubMed
-
- Agnello V, Ábel G. Arthritis Rheum. 1997;40:2007–2015. - PubMed
-
- Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, Pietrogrande M, Renoldi P, Bombardieri S, Bordin G. Q J Med. 1995;88:115–26. - PubMed
-
- Thomssen R, Bonk S, Propfe C, Heermann K H, Kochel H G, Uy A. Med Microbiol Immunol. 1992;181:293–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

