Cardioprotection from ischemia by a brief exposure to physiological levels of ethanol: role of epsilon protein kinase C
- PMID: 10536000
- PMCID: PMC23099
- DOI: 10.1073/pnas.96.22.12784
Cardioprotection from ischemia by a brief exposure to physiological levels of ethanol: role of epsilon protein kinase C
Abstract
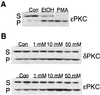
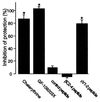
Recent epidemiological studies indicate beneficial effects of moderate ethanol consumption in ischemic heart disease. Most studies, however, focus on the effect of long-term consumption of ethanol. In this study, we determined whether brief exposure to ethanol immediately before ischemia also produces cardioprotection. In addition, because protein kinase C (PKC) has been shown to mediate protection of the heart from ischemia, we determined the role of specific PKC isozymes in ethanol-induced protection. We demonstrated that (i) brief exposure of isolated adult rat cardiac myocytes to 10-50 mM ethanol protected against damage induced by prolonged ischemia; (ii) an isozyme-selective epsilonPKC inhibitor developed in our laboratory inhibited the cardioprotective effect of acute ethanol exposure; (iii) protection of isolated intact adult rat heart also occurred after incubation with 10 mM ethanol 20 min before global ischemia; and (iv) ethanol-induced cardioprotection depended on PKC activation because it was blocked by chelerythrine and GF109203X, two PKC inhibitors. Consumption of 1-2 alcoholic beverages in humans leads to blood alcohol levels of approximately 10 mM. Therefore, our work demonstrates that exposure to physiologically attainable ethanol levels minutes before ischemia provides cardioprotection that is mediated by direct activation of epsilonPKC in the cardiac myocytes. The potential clinical implications of our findings are discussed.
Figures



References
-
- Parratt J R. Trends Pharmacol Sci. 1994;15:19–25. - PubMed
-
- De Labry L O, Glynn R J, Levenson M R, Hermos J A, LoCastro J S, Vokonas P S. J Stud Alcohol. 1992;53:25–32. - PubMed
-
- Ahlawat S K, Siwach S B. Int J Cardiol. 1994;44:157–162. - PubMed
-
- Langer R D, Criqui M H, Reed D M. Circulation. 1992;85:910–915. - PubMed
-
- Kobayashi H, Ashraf M, Rahamathulla P M, Minami M. Pathol Res Pract. 1987;182:810–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

