Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons
- PMID: 10536005
- PMCID: PMC23112
- DOI: 10.1073/pnas.96.22.12816
Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons
Abstract
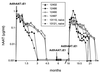
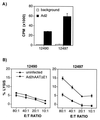
The efficiency of first-generation adenoviral vectors as gene delivery tools is often limited by the short duration of transgene expression, which can be related to immune responses and to toxic effects of viral proteins. In addition, readministration is usually ineffective unless the animals are immunocompromised or a different adenovirus serotype is used. Recently, adenoviral vectors devoid of all viral coding sequences (helper-dependent or gutless vectors) have been developed to avoid expression of viral proteins. In mice, liver-directed gene transfer with AdSTK109, a helper-dependent adenoviral (Ad) vector containing the human alpha(1)-antitrypsin (hAAT) gene, resulted in sustained expression for longer than 10 months with negligible toxicity to the liver. In the present report, we have examined the duration of expression of AdSTK109 in the liver of baboons and compared it to first-generation vectors expressing hAAT. Transgene expression was limited to approximately 3-5 months with the first-generation vectors. In contrast, administration of AdSTK109 resulted in transgene expression for longer than a year in two of three baboons. We have also investigated the feasibility of circumventing the humoral response to the virus by sequential administration of vectors of different serotypes. We found that the ineffectiveness of readministration due to the humoral response to an Ad5 first-generation vector was overcome by use of an Ad2-based vector expressing hAAT. These data suggest that long-term expression of transgenes should be possible by combining the reduced immunogenicity and toxicity of helper-dependent vectors with sequential delivery of vectors of different serotypes.
Figures

References
-
- Stratford-Perricaudet L D, Levrero M, Chase J F, Perricaudet M, Briand P. Hum Gene Ther. 1990;1:241–256. - PubMed
-
- Rosenfeld M A, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier L E, Pääkkö P K, Gilardi P, Stratford-Perricaudet L D, Perricaudet M, et al. Science. 1991;252:431–434. - PubMed
-
- Jaffe H, Danel C, Longenecker G, Metzger M, Setoguchi Y, Rosenfeld M A, Gant T W, Thorgeirsson S S, Stratford-Perricaudet L D, Perricaudet M, et al. Nat Genet. 1992;1:372–378. - PubMed
-
- Hitt M, Addison C, Graham F L. In: Advances in Pharmacology-Gene Therapy. August J T, editor. Vol. 40. San Diego: Academic; 1997. pp. 137–206. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

