Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos
- PMID: 10536026
- PMCID: PMC23170
- DOI: 10.1073/pnas.96.22.12935
Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos
Abstract
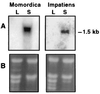
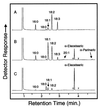
Vegetable oils that contain fatty acids with conjugated double bonds, such as tung oil, are valuable drying agents in paints, varnishes, and inks. Although several reaction mechanisms have been proposed, little is known of the biosynthetic origin of conjugated double bonds in plant fatty acids. An expressed sequence tag (EST) approach was undertaken to characterize the enzymatic basis for the formation of the conjugated double bonds of alpha-eleostearic (18:3Delta(9cis, 11trans,13trans)) and alpha-parinaric (18:4Delta(9cis,11trans, 13trans,15cis)) acids. Approximately 3,000 ESTs were generated from cDNA libraries prepared from developing seeds of Momordica charantia and Impatiens balsamina, tissues that accumulate large amounts of alpha-eleostearic and alpha-parinaric acids, respectively. From ESTs of both species, a class of cDNAs encoding a diverged form of the Delta(12)-oleic acid desaturase was identified. Expression of full-length cDNAs for the Momordica (MomoFadX) and Impatiens (ImpFadX) enzymes in somatic soybean embryos resulted in the accumulation of alpha-eleostearic and alpha-parinaric acids, neither of which is present in untransformed soybean embryos. alpha-Eleostearic and alpha-parinaric acids together accounted for as much as 17% (wt/wt) of the total fatty acids of embryos expressing MomoFadX. These results demonstrate the ability to produce fatty acid components of high-value drying oils in transgenic plants. These findings also demonstrate a previously uncharacterized activity for Delta(12)-oleic acid desaturase-type enzymes that we have termed "conjugase."
Figures


References
-
- Smith C R., Jr . In: Progress in the Chemistry of Fats and Other Lipids. Holman R T, editor. Vol. 11. London: Pergammon; 1970. pp. 137–177.
-
- Conacher H B S, Gunstone F D, Hornby G M, Padley F B. Lipids. 1970;5:434–441.
-
- Gunstone F D, Subbarao R. Chem Phys Lipids. 1967;1:349–359.
-
- Bagby M O, Smith C R, Jr, Wolff I A. Lipids. 1966;1:263–267. - PubMed
-
- Formo M W. In: Bailey’s Industrial Oil and Fat Products. Swern D, editor. New York: Wiley; 1979. pp. 708–709.
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

