The long-term effects of placebo in patients with chronic schizophrenia
- PMID: 10536745
- PMCID: PMC4303345
- DOI: 10.1016/s0006-3223(99)00227-9
The long-term effects of placebo in patients with chronic schizophrenia
Abstract
Background: It has been hypothesized that placebo periods may increase long-term morbidity for patients with schizophrenia. In this study, the long-term effect of a placebo period was evaluated in a group of relatively treatment-refractory patients with chronic schizophrenia.
Methods: This retrospective study examined behavioral rating scores for 127 patients with chronic schizophrenia who were placed in a double-blind placebo study on the inpatient units of the National Institute of Mental Health Neuropsychiatric Research Hospital. Patients were rated daily with the Psychiatric Symptom Assessment Scale (PSAS), an extended and anchored version of the Brief Psychiatric Rating Scale (BPRS). At the end of the placebo phase, most patients were placed on haloperidol. Pre-placebo baseline PSAS ratings were compared with, first, discharge ratings and second, post-placebo ratings. To determine expected variability in the course of illness, patients in the placebo group were compared with patients hospitalized during the same time period, but who did not enter the placebo study.
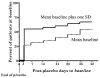
Results: By discharge, ratings for placebo patients had returned to baseline. Post-placebo ratings were quite variable. Although many of the placebo patients had returned to baseline by day 3 of the post-placebo phase, others had not returned to baseline by post-placebo day 42. PSAS Total Scores for patients who left the study early were no different at baseline, placebo, or through post-placebo day 35 compared with patients who completed the study.
Conclusions: The results indicate that given a sufficiently lengthy recovery period, patients with chronic schizophrenia who go through a placebo phase return to baseline, but that the speed with which they attain that recovery is highly variable.
Figures
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-III. 3. Washington, D.C: The American Psychiatric Association; 1980.
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-III-R. 3. Washington, D.C: The American Psychiatric Association; 1987. revised.
-
- Angst J. European long-term followup studies of schizophrenia. Schizophr Bull. 1988;14:501–513. - PubMed
-
- Applebaum P. Rethinking the conduct of psychiatric research. Arch Gen Psychiatry. 1997;54:117–120. - PubMed
-
- Applebaum PS. Drug-free research in schizophrenia: an overview of the controversy. IRB. 1996;18:1–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical