Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse
- PMID: 10537162
- PMCID: PMC4280800
- DOI: 10.1210/endo.140.11.7148
Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse
Abstract
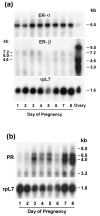
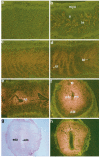
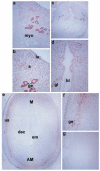
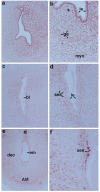
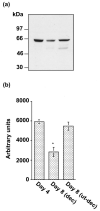
The present investigation examined the spatiotemporal expression of estrogen receptors (ER-alpha and ER-beta) and progesterone receptor (PR) in the periimplantation mouse uterus (days 1-8). ER-alpha messenger RNA (mRNA) was detected at much higher levels in the periimplantation uterus compared with that of ER-beta mRNA, the levels of which were very low in all uterine cells during this period. Results of in situ hybridization demonstrated expression of ER-alpha mRNA primarily in the luminal and glandular epithelia on days 1 and 2 of pregnancy. On days 3 and 4, the accumulation was localized primarily in stromal cells in addition to its presence in the epithelium. Following implantation on day 5, the accumulation of this mRNA was more condensed in the luminal and glandular epithelia, but declined in the subluminal epithelial stroma at the sites of implanting embryos. On days 6-8, the accumulation of ER-alpha mRNA was primarily localized in the secondary decidual zone (SDZ) with more intense localization in the subepithelial cells at the mesometrial pole. In contrast, signals were very low to undetectable in the primary decidual zone (PDZ), and no signals were detected in implanting embryos. The undifferentiated stroma underneath the myometrium also showed positive signals. The immunolocalization of ER-alpha protein correlated with the mRNA localization. Western blot analysis showed down-regulation of ER-alpha in day 8 decidual cell extracts consistent with the down-regulation of ER-alpha mRNA in decidual cells immediately surrounding the embryo on this day. The expression pattern of PR was also dynamic in the periimplantation uterus. On day 1, the accumulation of PR mRNA was very low to undetectable, whereas only a modest level of accumulation in the epithelium was noted on day 2. On days 3 and 4, the accumulation of this mRNA was detected in both the epithelium and stroma. In contrast, the expression was restricted only to the stroma with increased signals at the sites of implantation on day 5. On days 6-8, PR mRNA accumulation increased dramatically throughout the deciduum. The localization of immunoreactive PR correlated with the mRNA distribution in the periimplantation uterus. Taken together, the results demonstrate that the expression of ER-alpha, ER-beta, and PR is differentially regulated in the periimplantation mouse uterus. This compartmentalized expression of ER and PR provides information regarding the sites of coordinated effects ofestrogen and progesterone in the preparation of the uterus for implantation and decidualization during early pregnancy.
Figures


References
-
- Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after estrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–144. - PubMed
-
- Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-Myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–1690. - PubMed
-
- Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. - PubMed
-
- Psychoyos A. Endocrine control of egg implantation. In: Greep RO, Astwood EG, Geiger SR, editors. Handbook of Physiology. American Physiological Society; Washington DC: 1973. pp. 187–215.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

